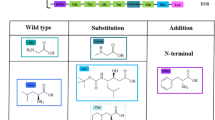
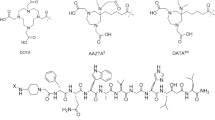
Abstract
Treatment with radionuclide labeled regulatory peptides is a promising tool in the management of patients with inoperable receptor positive neuroendocrine tumors. Peptide receptor lutetium-177 radionuclide therapy currently has gained ample attention due to high specific accumulation of regulatory peptides at tumor cell surface and promising characteristics of β- and γ-energy photons of lutetium-177 radionuclide. In this study gastrin peptides analogues were labeled with lutetium-177 by subsequent mixing of 177LuCl3 (~ 185 MBq), ammonium acetate buffer of 5 pH, gentistic acid, aqueous solution of gastrin peptide analogues (1 mg/mL) and heating the reaction mixture at 98 °C which resulted in high radiochemical yield (> 96%). Chromatographic analysis was carried out to analyze the radiochemical purity. The shelf life and serum stability results showed the labeled peptides are sufficiently stable up to 4-h. Glomerular filtration rate study results showed moderate filtration through kidneys. The GFR values of 177Lu-MGCL2 and 177Lu-MGCL4 was noted 48 mL/min and 45 mL/min, respectively. Biodistribution and scintigraphy study using rat and rabbit models showed minimal non-target accumulation, moderate uptake by liver and kidneys. The promising radiochemical yield, stability, GFR values and biodistribution results of 177Lu-MGCL2 & 4 indicate, the agents can be tested clinically for PRRT procedures.







Similar content being viewed by others
References
Reubi JC (2003) Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24:389–427
Kobilka BK (2007) G protein coupled receptor structure and activation. Biochimica et Biophysica Acta (BBA) 1768:794–807
Naqvi SA, Matzow T, Finucane C, Nagra SA, Ishfaq MM, Mather SJ, Sosabowski J (2010) Insertion of a lysosomal enzyme cleavage site into the sequence of a radiolabeled neuropeptide influences cell trafficking in vitro and in vivo. Cancer Biother Radiopharm 25:89–95
Fani M, Maecke HR, Okarvi SM (2012) Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics 2:481–501
Grider JR (1994) Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr 124:1334S–1339S
Nock BA, Maina T, Béhé M, Nikolopoulou A, Gotthardt M, Schmitt JS, Behr TM, Mäcke HR (2005) CCK-2/gastrin receptor–targeted tumor imaging with 99mTc-labeled minigastrin analogs. J Nucl Med 46:1727–1736
Brans B, Linden O, Giammarile F, Tennvall J, Punt C (2006) Clinical applications of newer radionuclide therapies. Eur J Cancer 42:994–1003
Dash A, Chakraborty S, Pillai MR, Knapp FF Jr (2015) Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm 30:47–71
Laverman P, Sosabowski JK, Boerman OC, Oyen WJG (2012) Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging 39:78–92
Mather SJ, McKenzie AJ, Sosabowski JK, Morris TM, Ellison D, Watson SA (2007) Selection of radiolabeled gastrin analogs for peptide receptor–targeted radionuclide therapy. J Nucl Med 48:615–622
Accardo A, Galli F, Mansi R, Del Pozzo L, Aurilio M, Morisco A, Ringhieri P, Signore A, Morelli G, Aloj L (2016) Pre-clinical evaluation of eight DOTA coupled gastrin-releasing peptide receptor (GRP-R) ligands for in vivo targeting of receptor-expressing tumors. EJNMMI Res 6:17
Jamous M, Haberkorn U, Mier W (2013) Synthesis of peptide radiopharmaceuticals for the therapy and diagnosis of tumor diseases. Molecules 18:3379–3409
Vegt E, de Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, Gotthardt M, Boerman OC (2010) Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med 51:1049–1058
Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, Modification of Diet in Renal Disease Study Group, The Diabetes Control and Complications Trial Research Group (1993) Glomerular filtration rate measurements in clinical trials. J Am Soc Nephrol 4:1159–1171
Rasheed R, Tariq S, Naqvi SA, Gillani SJ, Rizvi FA, Sajid M, Rasheed S (2016) Lu-5-Fluorouracil a potential theranostic radiopharmaceutical: radiosynthesis, quality control, biodistribution, and scintigraphy. J Label Comp Radiopharm 59(177):398–403
Fani M, Peitl P, Velikyan I (2017) Current status of radiopharmaceuticals for the theranostics of neuroendocrine neoplasms. Pharmaceuticals 10:30
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M, Paganelli G (2008) Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35:1847–1856
Behr TM, Behe M, Angerstein C, Gratz S, Mach R, Hagemann L, Jenner N, Stiehler M, Frank-Raue K, Raue F, Becker W (1999) Cholecystokinin-B/gastrin receptor binding peptides: preclinical development and evaluation of their diagnostic and therapeutic potential. Clin Cancer Res 5:3124s–3138s
Dash A, Chakraborty S, Pillai MRA, Knapp FF (2015) Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm 30:47–71
Dash A, Pillai MR, Knapp FF Jr (2015) Production of (177)Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging 49:85–107
Knapp FF Jr, Mirzadeh S, Beets AL, Du M (2005) Production of therapeutic radioisotopes in the ORNL High Flux Isotope Reactor (HFIR) for applications in nuclear medicine, oncologyand interventional cardiology. J Radioanal Nucl Chem 263:503–509
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BLR, Teunissen JJM, Kooij PP, Mauff KAL, Krenning EP, Kwekkeboom DJ (2016) Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging 43:1802–1811
Mohamed MM, Sloane BF (2006) Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 6:764–775
Emami B (1999) Three-dimensional conformal radiotherapy in treatment of bronchogenic carcinoma. In: Van Houtte P (ed) Progress and perspective in the treatment of lung cancer. Springer, Berlin, pp 109–117
Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ (2017) Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 23:4617–4624
Acknowledgements
The authors are highly grateful to Prof. Dr. Stephen John Mather for his countless assistance in this study and Higher Education Commission (HEC), Islamabad, Pakistan for funding (Project No. 3413/NRPU/HEC/R&D/14/702). The authors are also thankful to the Dr. Jawad Akhtar Hussain Gillani (Nuclear Oncologist), Institute of Nuclear Medicine Oncology and Radiotherapy (INOR), Abbottabad for providing platform for labeling and SPECT scintigraphy study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article is in compliance with ethical standards with approval from ethical review committee.
Informed consent
Informed consent was taken from all co-authors before submission of manuscript.
Rights and permissions
About this article
Cite this article
Rizvi, S.F.A., Naqvi, S.A.R., Roohi, S. et al. 177Lu-DOTA-coupled minigastrin peptides: promising theranostic agents in neuroendocrine cancers. Mol Biol Rep 45, 1759–1767 (2018). https://doi.org/10.1007/s11033-018-4319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4319-0