Abstract
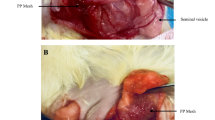
In the present study, effects on expression of antioxidant, apoptotic and anti-apoptotic genes (GSR, GRX3, SOD1, RAI-NOS, HSP7, BAX, Bcl-2, CASP3 and MDH1) of substances being used in non-surgical sterilization such as quinacrine, erythromycin and tetracycline were evaluated in over tissue. Moreover, expression of some specific mi-RNA (miR-15b, miR-21, miR34a and miR-98) that playing a role in apoptosis was determined in same tissue. Prospective comparative experimental study. Genetics and Histology laboratory. Total number of 28 Wistar albino 12–14 week old female rats with regular cycles and 200–220 grams in weight. Total RNA was isolated from tissues by using a RNA isolation kit. Gene expression levels were evaluated by Real-Time PCR method. Tubal passage and fibrosis induction in tissues was observed in the histochemical analysis. In the statistical analysis of data Kruskal–Wallis variance analysis and Mann–Whitney U test were used and p < 0.05 were accepted as significant. While the expressions of target genes found to be increased in quinacrine and erythromycin group when compared to control group, this increase was insignificant. In quinacrine group, increase in the SOD1 expression levels was only statistically significant (p < 0.05). Expression levels of miR-15b, miR-21, miR34a and miR-98 microRNAs were found to be up-regulated in all experimental groups, despite this, only the increased expression miR-34 was found as statistically significant when compared to control. Tubal blockage and fibrosis induction scores of quinacrine, erythromycin and tetracycline were significantly higher than control. Results of the present study suggest that the doses treated of quinacrine, erythromycin and tetracycline used in non-surgical sterilization effect poorly the expression of anti-oxidant, apoptotic and anti-apoptotic genes, but the expression of miR-34 playing the role in apoptosis increased after treatment of these substances.
Similar content being viewed by others
References
Chamberlain G (1978) Confidential inquiry into gynaecological laparoscopy. Br Med J 19:563
Peterson HB, Hulka JF, Phillips JM, Surrey MW (1993) Laparoscopic sterilization. American Association of Gynecologic Laparoscopists 1991 membership survey. Reprod Med 38:574–576
Lippes J (2002) Quinacrine sterilization: the imperative need for american clinical trials. Fertil Steril 77:1106–1109
Zipper J, Cole LP, Goldsmith A, Wheeler R, Rivera M (1980) Quinacrine hydrochloride pellets: preliminary data on a nonsurgical method of female sterilization. Int J Gynaecol Obstet 18:275–279
Bhuiyan SN, Begum R (2001) Quinacrine non-surgical female sterilization in Bangladesh. Contraception 64:281–286
Mullick B, Mumford SD, Kessel E (1987) Studies of quinacrine and of tetracyclinefornon-surgicalfemalesterilization. AdvContracept 3:245–254
Fail PA, Martin P, Sokal D (2000) Comparative effects of quinacrine and erythromycin in adult female rats: a nonsurgical sterilization study. Fertil Steril 73:387–394
Bairagy NR, Mullick BC (2004) Use of erythromycin for nonsurgical female sterilization in West Bengal, India: a study of 790 cases. Contraception 69:47–49
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S et al (2009) MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10:1252–1259
Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW et al (2009) MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol 183:1617–1624
Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H et al (2011) MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 23:1963–1968
Finnerty JR, Wang WX, Hébert SS, Wilfred BR, Mao G, Nelson PT (2010) The miR-15/107 group of microRNA genes: evolutionary biology. Cellular functions and roles in human diseases. J Mol Biol 402:491–509
Yan-nan B, Zhao-yan Y, Li-xi L, Jiang L, Qing-jie X, Yong Z (2014) MicroRNA-21accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN. Biochem Biophys Res Commun 443:802–807
Rokavec M, Li H, Jiang L, Hermeking H (2014) The p53/miR-34 axis in development and disease. J Mol Cell Biol 6:214–230
Mallenby J, Dunyer J, Hawkins C, Hitchen C (1991) Effects of experimental limbic on the estrus cycle and reproductive success in rats. Epilepsia 34:220–227
Hooker GD, Taylor BM, Driman DK (1999) Prevention of adhesion formation with use of sodium hyaluronate-based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh. A randomized controlled study. Surgery 125:211–216
Mitra SN, Ahmed A-S, Cross AR, Jamil K (1997) Bangladesh demographic and health survey, 1996–1997. National Institute of Population Research and Training (NIPORT). Mitra and Associates and Macro International Inc., Calverton
Liochev SI (2013) Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 60:1–4
Sun Y (1990) Free radicals antioxidant enzymes and carcinogenesis. Free Radic Biol Med 8:583–599
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70:257–265
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 277:755–776
Mraz M, Malinova K, Kotaskova J, Pavlova S, Tichy B, Malcikova J et al (2009) MiR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia 23:1159–1163
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105:13421–13426
Welch C, Chen Y, Stallings RL (2007) MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26:5017–5022
Yu M, Mu H, Niu Z et al (2014) miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J Cell Biochem 115:232–242
Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N et al (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743
Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z et al (2012) MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor retinoblastoma protein (E2F-pRb) pathway. J Biol Chem 287:21686–21698
Soni K, Choudhary A, Patowary A, Singh AR, Bhatia S, Sivasubbu S et al (2013) miR-34 is maternally inherited in Drosophila melanogaster and Danio rerio. Nucleic Acids Res 41:4470–4480
Merchant RN, Prabhu SR, Kessel E (1995) Clinicopathologic study of fallopian tube closure after single transcervical insertion of quinacrine pellets. Int J Fertil Menopausal Stud 40:47–54
Conflict of interest
There is no potential source of conflict to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kara, M., Yumrutas, O., Atilgan, R. et al. Expression changes of antioxidant, apoptotic, anti-apoptotic genes and miR-15b-34a-21-98 in over tissue by using erythromycin, quinacrine and tetracycline in non-surgical sterilization. Mol Biol Rep 41, 8093–8098 (2014). https://doi.org/10.1007/s11033-014-3707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3707-3