Abstract
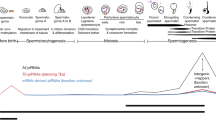
Piwi-interacting RNAs (piRNAs) play a role in gene silencing of retrotransposons, maintenance of spermatogenesis and maturation in germlines. The piRNA and PIWI protein are essential for fertility. To reveal piRNA function associated with testosterone, we investigated the expression of piRNA and piwi protein in normal male rats and testosterone-treated rats. Normal Sprague–Dawley (SD) rats were randomly selected and sacrificed at neonatal to late adolescence stage stages (2, 9, 16, 20, 24, 28, 35, and 42 days, n = 6 each). Additional SD rats were divided into four groups: group 1 received weekly injections of testosterone enanthate (8 mg/100 g) during 1–3 weeks; group 2 during 3–5 weeks; group 3 during 1–5 weeks; and group 4 was the control (n = 20 each). These animals were sacrificed at an age of 60 days. We investigated piRNA, PIWI, and Ago3 protein levels using real-time PCR, Western blot, and immunohistochemistry in each group. In normal rats, PIWI protein and piRNA were expressed at P24. The expression of PIWI and piRNA gradually increased from adolescence to adulthood on Western blot, real-time PCR and immunohistochemistry. In testosterone-treated rats, the expression of PIWI protein was analyzed by Western blot and shown to be significantly increased in group 1 (neonatal to juvenile injection). In real-time PCR, the expression of piRNA after testosterone treatment was increased in all groups (G1 166.8 ± 2.7; G2 113.3 ± 4.6; G3 70.2 ± 1.5 vs. control, 32.87 ± 2.0, all p < 0.001). The expression of testosterone in adolescence induces the development of male genitourinary organs and spermatogenesis. At the same time, the sexual hormones may activate the piRNA and PIWI protein. Our data demonstrate that patterns of piRNA and PIWI expression are similar to the secretion pattern of testosterone, and that piRNA expression was increased after testosterone treatment. Therefore, testosterone may affect testis function through the regulation of piRNA expression in rats.




Similar content being viewed by others
References
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Li C, Vagin VV, Lee S et al (2009) Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137:509–521
Nagao A, Mituyama T, Huang H, Chen D, Siomi MC, Siomi H (2010) Biogenesis pathways of piRNAs loaded onto AGO3 in the Drosophila testis. RNA 16:2503–2515
Kim VN (2006) Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev 20:1993–1997
Lin H (2007) piRNAs in the germ line. Science 316:397
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Miyoshi K, Miyoshi T, Hartig JV, Siomi H, Siomi MC (2010) Molecular mechanisms that funnel RNA precursors into endogenous small-interfering RNA and microRNA biogenesis pathways in Drosophila. RNA 16:506–515
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Mourier T (2011) Retrotransposon-centered analysis of piRNA targeting shows a shift from active to passive retrotransposon transcription in developing mouse testes. BMC Genomics 12:440
Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD (2006) A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313:320–324
Houwing S, Kamminga LM, Berezikov E et al (2007) A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 129:69–82
Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ (2007) Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316:744–747
Carmell MA, Girard A, van de Kant HJG, Bourc’his D, Bestor TH, de Rooij DG, Hannon GJ (2007) MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12:503–514
Handler D, Olivieri D, Novatchkova M, Gruber FS, Meixner K, Mechtler K, Stark A, Sachidanandam R, Brennecke J (2011) A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J 30:3977–3993
Cox DN, Chao A, Lin H (2000) piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127:503–514
Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H (1998) A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev 12:3715–3727
Klattenhoff C, Theurkauf W (2008) Biogenesis and germline functions of piRNAs. Development 135:3–9
Lau NC, Robine N, Martin R, Chung WJ, Niki Y, Berezikov E, Lai EC (2009) Abundant primary piRNAs, endo-siRNAs, and microRNAs in a Drosophila ovary cell line. Genome Res 19:1776–1785
Shalet SM (2009) Normal testicular function and spermatogenesis. Pediatr Blood Cancer 53:285–288
Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4:624–630
Tang F, Hayashi K, Kaneda M, Lao K, Surani MA (2008) A sensitive multiplex assay for piRNA expression. Biochem Biophys Res Commun 369:1190–1194
Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, Kosik KS (2011) Identification of piRNAs in the central nervous system. RNA 17:1090–1099
Saito K, Inagaki S, Mituyama T, Kawamura Y, Ono Y, Sakota E, Kotani H, Asai K, Siomi H, Siomi MC (2009) A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila. Nature 461:1296–1299
Beyret E, Lin H (2011) Pinpointing the expression of piRNAs and function of the PIWI protein subfamily during spermatogenesis in the mouse. Dev Biol 355:215–226
Deng W, Lin H (2002) miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2:819–830
Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z (2009) Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol 11:652–658
Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC (2007) A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315:1587–1590
Gaidano G, Berta L, Rovero E, Valenzano C, Rosatti P (1980) Dynamics of the binding capacity of plasma sex hormone binding globulin (SHBG) for testosterone and dihydrotestosterone during puberty. Clin Chim Acta 100:91–97
Purves-Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS (2012) Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci 13:95
Lee H, Jin MH, Kang HJ, Hong CH, Bang WJ, Park KK, Han SW (2010) Timing of prepubertal androgen administration may have different effects on future fertility as well as penile size in normal male rats. Urology 75:979–984
Dunkel L, Taskinen S, Hovatta O, Tilly JL, Wikstrom S (1997) Germ cell apoptosis after treatment of cryptorchidism with human chorionic gonadotropin is associated with impaired reproductive function in the adult. J Clin Invest 100:2341–2346
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, H.J., Moon, M.J., Lee, H.Y. et al. Testosterone alters testis function through regulation of piRNA expression in rats. Mol Biol Rep 41, 6729–6735 (2014). https://doi.org/10.1007/s11033-014-3558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3558-y