Abstract
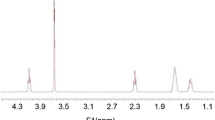
In the recent years, temperature and pH-sensitive hydrogels were developed as suitable carriers for drug delivery. In this study, four different pH-sensitive nanohydrogels were designed for an oral insulin delivery modeling. NIPAAm–MAA–HEM copolymers were synthesized by radical chain reaction with 80:8:12 ratios respectively. Reactions were carried out in four conditions including 1,4-dioxan and water as two distinct solution under nitrogen gas-flow. The copolymers were characterized with FT-IR, SEM and TEM. Copolymers were loaded with regular insulin by modified double emulsion method with ratio of 1:10. Release study carried out in pH 1.2 and pH 6.8 at 37 °C. For pH 6.8 and pH 1.2, 2 mg of the insulin loaded nanohydrogels was float in a beaker containing 100 mL of PBS with pH 6.8 and 100 mL of HCl solution with pH 1.2, respectively. Sample collection was done in different times and HPLC was used for analysis of samples using water/acetonitrile (65/35) as the mobile phase. Nanohydrogels synthesis reaction yield was 95 %, HPLC results showed that loading in 1,4-dioxan without cross-linker nanohydrogels was more than others, also indicated that the insulin release of 1,4-dioxan without cross-linker nanohydrogels at acidic pH is less, but in pH 6.8 is the most. Results showed that by opting suitable polymerization method and selecting the best nanohydrogels, we could obtain a suitable insulin loaded nanohydrogels for oral administration.





Similar content being viewed by others
References
Morçöl T, Nagappan P, Nerenbaum L, Mitchell A (2004) Calcium phosphate–PEG–insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int J Pharm 277(1–2):91–97
Sweetman S (ed) (2007) Martindale, the complete drug reference. Pharmaceutical Press, London Electronic version
King H, Aubert RE, Herman WH (1998) Global burden of diabets 1995–2005: prevalence, numerical, estimates and projection. Diabets Care 21:1414–1431
Akhter DT, Nijhu RS (2012) Diabetes mellitus: a journey of insulin. Int Curr Pharm J 1(2):32–42
Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA (2006) Novel oral insulin delivery systems based on complexation polymer hydrogels: single and multiple administration studies in type 1 and 2 diabetic rats. J Control Release 110:587–594
Kinesh VP, Neelam DP, Punit BP, Bhavesh SB, Pragna KS (2010) Novel approaches for oral delivery of insulin and current status of oral insulin products. Int J Pharm Sci Nanotechnol 3(3):1057–1064
Davaran S, Jafari B, Rafie F (2011) Pharmaceutical sciences, oral insulin: a review on its current condition and future aspects. Pharm Sci 17(3):151–162
Carino GP, Mathiowitz E (1999) Oral insulin delivery. J Adv Drug Deliv Rev 35:249–257
Pan YJ, Chen YY, Wang DR, Wei Ch, Guo J, Lu DR, Chu CC, Wang CC (2012) Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 33(27):6570–6579
Lowman AM (1999) Oral delivery of insulin using pH sensitive complexation gels. J Pharm Sci 88(9):933–937
Peppas NA (2004) Devices based on intelligent biopolymers for oral protein delivery. Int J Pharm 277:11–17
Ramkisson-Ganorkar C (1999) Modulating insulin release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J Control Release 59(3):287–298
Reis CP, Ribeiro AJ, Neufeld RJ, Veiga F (2007) Alginate microparticles as novel carrier for oral insulin delivery. J Biotechnol Bioeng 96(5):97
Gupta S, Kuckling D, Kretschmer K, Choudhary V, Rgen Adler H (2006) Synthesis and characterization of stimuli-sensitive micro- and nanohydrogels based on photocrosslinkable poly(dimethylaminoethyl methacrylate). J Polym Sci Part A Polym Chem. doi:10.1002/pola.21846
Akiyosh K, Kobayashi S, Schichibe S, Mix D, Baudys M, Kim SW, Sunamoto J (1998) Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J. Control Release 54(3):313–320
Na K, Bae YH (2004) Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: characterization, aggregation and adriamycin release in vitro. Pharm Res 19(5):681–688
Argentiere S, Blasi L, Ciccarella G, Barbarella G, Cingolani R, Gigli G (2010) Nanogels of poly (acrylic acid): uptake and release behavior with fluorescent oligothiophene-labeled bovine serum albumin. J Appl Polym Sci 116(5):2808–2815
Liu KH, Liu TY, Chen SY, Liu DM (2008) Drug release behavior of chitosan–montmorillonite nanocomposite hydrogels following electrostimulation. Acta Biomater 4:1038–1045
Sahiner N, Godbey WT, McPherson GL, John VT (2006) Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: synthesis and characterization. Colloid Polym Sci 284:1121–1129. doi:10.1007/s00396-006-1489-4
Üzüm Ö, Karadağ E (2007) Swelling characterization of poly (acrylamide-co-N-vinylimidazole) hydrogels crosslinked by TMPTA and semi-IPN’s with PEG. J Polym Res 14:483–488
Jafari B, Rafie F, Davaran S (2011) Preparation and characterization of a novel smart polymeric hydrogel for drug delivery of insulin. Bio Impacts 1(2):135–143
Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Higginbotham CL (2006) The synthesis, characterisation, phase behaviour and swelling of temperature sensitive physically crosslinked poly (1-vinyl-2-pyrrolidinone)/poly (N-isopropylacrylamide) hydrogels. Eur Polym J 42(1):69–80
Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK (2002) Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials 23:457–462
Hsiue GH, Chang RW, Wang CH, Lee SH (2003) Development of in situ thermosensitive drug vehicles for glaucoma therapy. Biomaterials 24:2423–2430
Matsumaru Y, Hyodo A, Nose T, Ito S, Hirano T, Ohashi S (1996) Application of thermosensitive polymers as a new embolic material for intravascular neurosurgery. J Biomater Sci Polym Ed 7:795–804
Lee BH, Leon C, McLemore R, Macias JV, Vernon BL (2011) Synthesis and characterization of thermo-sensitive radio-opaque poly (N isopropylacrylamide-co-PEG-2-iodobenzoate). J Biomater Sci Polym Ed 22:2357–2367
Acknowledgments
The authors thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all supports provided. This work was funded by 2013 Drug Applied Research Center Grant.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karnoosh-Yamchi, J., Mobasseri, M., Akbarzadeh, A. et al. Preparation of pH sensitive insulin-loaded nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol Biol Rep 41, 6705–6712 (2014). https://doi.org/10.1007/s11033-014-3553-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3553-3