Abstract
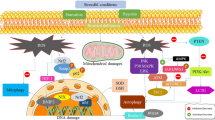
Reactive oxygen species (ROS) are produced due to oxidative stress which has wide range of affiliation with different diseases including cancer, heart failure, diabetes and neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, ischemic and hemorrhagic diseases. This study shows the involvement of BNIP3 in the amplification of metabolic pathways related to cellular quality control and cellular self defence mechanism in the form of autophagy. We used conventional methods to induce autophagy by treating the cells with H2O2. MTT assay was performed to observe the cellular viability in stressed condition. MDC staining was carried out for detection of autophagosomes formation which confirmed the autophagy. Furthermore, expression of BNIP3 was validated by western blot analysis with LC3 antibody. From these results it is clear that BNIP3 plays a key role in defence mechanism by removing the misfolded proteins through autophagy. These results enhance the practical application of BNIP3 in neuroblastoma cells and are helpful in reducing the chances of neurodegenerative diseases. Although, the exact mode of action is still unknown but these findings unveil a molecular mechanism for the role of autophagy in cell death and provide insight into complex relationship between ROS and non-apoptotic programmed cell death.




Similar content being viewed by others
References
Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate prone proteins with polyglutamine and polyalanine expansions is degraded by autophagy. Hum Mol Genet 11:1107–1117
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Lee JA, Gao FB (2008) Regulation of Abeta pathology by beclin 1: a protective role for autophagy. J Clin Invest 118:2015–2018
Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GR (2009) Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci 116:697–712
Tinsley RB, Bye CR, Parish CL, Tziotis-Vais A, George S, Culvenor JG (2009) Dopamine D2 receptor knockout mice develop features of Parkinson disease. Ann Neurol 66:472–484
Nicoletta Plotegher and Laura Civiero (2012) Neuronal Autophagy, α-Synuclein Clearance, and LRRK2 Regulation: a Lost Equilibrium in Parkinsonian Brain. J Neurosci 32:14851–14853
Gustafsson Asa B (2011) BNIP3 as a dual regulator of mitochondrial turnover and cell death in the myocardium. Pediatr Cardiol 32:267–274
Lomonosova E, Chinnadurai G (2008) BH3-only proteins in apoptosis and beyond: an overview. Oncogene 27:S2–S19
Burton TR, Gibson SB (2009) the role of Bcl-2 family member BNIP3 in cell death and disease: NIPping at the heels of cell death. Cell Death Differ 16:515–523
He C, Klionsky DJ (2009) Regulation mechanisms and signalling pathways of autophagy. Annul Rev Genet 43:67–93
Wang RC, Levine B (2010) Autophagy in cellular growth control. FEBS Lett 584:1417–1426
Tracy Kristin (2007) BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27:6229–6242
García-Arencibia M, Hochfeld WE, Toh PPC, Rubinsztein DC (2010) Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol 21:691–698
Essick EE, Sam F (2010) Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev Landes Biosci 3:168–177
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23:2891–2906
Trachtenberg BH, Hare JM (2009) Biomarkers of oxidative stress in heart failure. Heart Fail Clin 5:561–577
Biederbick A, Kern HF, Elsasser HP (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol 66:3–14
Swiderek E, Kalas W, Wysokinska E, Pawlak A, Rak J, Strzadala L (2013) The interplay between epigenetic silencing, oncogenic KRas and HIF-1 regulatory pathways in control of BNIP3 expression in human colorectal cancer cells. Biochem Biophys Res Commun 441:707–712
Chen J, Small-Howard A, Yin A, Berry MJ (2005) The responses of Ht22 cells to oxidative stress induced by buthionine sulfoximine (BSO). BMC Neurosci 6:10
Suzuki YJ, Forman HJ, Sevanian A (1997) Oxidants as stimulators of signal transduction. Free Radic Biol Med 22:269–285
Lee Y, Lee HY, Hanna RA, Gustafsson ÅB (2011) Mitochondrial autophagy by BNIP3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 301:H1924–H1931
Troyano A, Sancho P, Ferna´ndez C, de Blas E, Bernardiand P, Aller P (2003) The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathionedepleted human promonocytic cells. Cell Death Differ 10:889–898
Lennon SV, Martin SJ, Cotter TG (1991) Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif 24:203–214
Dypbukt JM, Ankarcrona M, Burkitt M, Sjöholm A, Ström K, Orrenius S, Nicotera P (1994) Different pro oxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem 269:30553–30560
Swiderek E, dała L Strza (2013) Autophagy and BNIP3 protein in tumorogenesis. Postepy Hig Med Dosw 67:363–370
Burton TR, Henson ES, Azad MB et al (2013) BNIP3 acts as transcriptional repressor of death receptor-5 expression and prevents TRAIL-induced cell death in gliomas. Cell Death Dis 4:e587
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12:814–822
De Duve C, Wattiaux R (1966) Functions of lysosomes. Annu Rev Physiol 28:435–492
kaza N (2012) Autophagy in brain tumors: a new target for therapeutic intervention. Brain Pathol 22:89–98
Pirtoli L, Cevenini G, Tini P, Vannini M, Oliveri G, Marsili S, Mourmouras V, Rubino G, Miracco C (2009) The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy 5:930–936
Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, Gustafsson AB (2011) BNIP3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ 18:721–731
Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson ÅB (2012) Microtubule-associated protein 1 light chain 3 (LC3) interacts with BNIP3 to selectively remove endoplasmic reticulum and Mitochondria via autophagy. J Biol Chem 287:1994–19104
Fan W, Nassiri A, Zhong Q (2011) Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc Natl Acad Sci USA 108:7769–7774
Thomas P (2012) Neufeld autophagy and cell growth—the yin and yang of nutrient responses. J Cell Sci 125:2359–2368
Neufeld TP (2004) Role of autophagy in developmental cell growth and death: insights from Drosophila. In: Klionsky DJ (ed) Autophagy. Landes Bioscience, Georgetown, pp 224–232
Lee Y, Lee HY, Hanna RA, Gustafsson AB (2011) Mitochondrial autophagy by BNIP3 involves Drp1-mediated mitochondrial fission and recruitment of parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 301:1924–1931
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Awan, M.U.F., Hasan, M., Iqbal, J. et al. Neuroprotective role of BNIP3 under oxidative stress through autophagy in neuroblastoma cells. Mol Biol Rep 41, 5729–5734 (2014). https://doi.org/10.1007/s11033-014-3444-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3444-7