Abstract
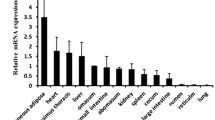
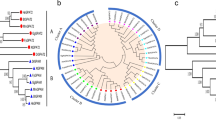
It has been demonstrated that ACSL3 and ACSL5 play important roles in fat metabolism. To investigate the primary functions of ACSL3 and ACSL5 and to evaluate their expression levels during goose fatty liver development, we cloned the ACSL3 and ACSL5 coding domain sequences (CDSs) of geese using RT-PCR and analyzed their expression characteristics under different conditions using qRT-PCR. The results showed that the goose ACSL3 (JX511975) and ACSL5 (JX511976) sequences have high similarities with the chicken sequences both at the nucleotide and amino acid levels. Both ACSL3 and ACSL5 have high expression levels in goose liver. The expression levels of ACSL3 and ACSL5 in goose liver and hepatocytes can be changed by overfeeding geese and by treatment with unsaturated fatty acids, respectively. Together, these results indicate that ACSL3 and ACSL5 play important roles during fatty liver development. The different expression characteristics of goose ACSL3 and ACSL5 suggest that these two genes may be responsible for specific functions.




Similar content being viewed by others
Abbreviations
- ACSL3:
-
Acyl-CoA synthetase long-chain family member 3
- ACSL5:
-
Acyl-CoA synthetase long-chain family member 5
- CDS:
-
Coding domain sequence
- TG:
-
Triglyceride
References
Digel M, Ehehalt R, Stremmel W, Fullekrug J (2009) Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol Cell Biochem 326:23–28. doi:10.1007/s11010-008-0003-3
Soupene E, Kuypers FA (2008) Mammalian long-chain acyl-CoA synthetases. Exp Biol Med (Maywood) 233:507–521. doi:10.3181/0710-MR-287
Coleman RA, Lewin TM, Muoio DM (2000) Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr 20:77–103. doi:10.1146/annurev.nutr.20.1.77
Bu SY, Mashek MT, Mashek DG (2009) Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J Biol Chem 284:30474–30483. doi:10.1074/jbc.M109.036665
Parkes HA, Preston E, Wilks D, Ballesteros M, Carpenter L, Wood L, Kraegen EW, Furler SM, Cooney GJ (2006) Overexpression of acyl-CoA synthetase-1 increases lipid deposition in hepatic (HepG2) cells and rodent liver in vivo. Am J Physiol Endocrinol Metab 291:E737–E744. doi:10.1152/ajpendo.0.0112.2006
Mashek DG, Bornfeldt KE, Coleman RA, Berger J, Bernlohr DA, Black P, DiRusso CC, Farber SA, Guo W, Hashimoto N, Khodiyar V, Kuypers FA, Maltais LJ, Nebert DW, Renieri A, Schaffer JE, Stahl A, Watkins PA, Vasiliou V, Yamamoto TT (2004) Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res 45:1958–1961. doi:10.1194/jlr.E400002-JLR200
Schoonjans K, Staels B, Auwerx J (1996) Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37:907–925
Zhou Y, Abidi P, Kim A, Chen W, Huang TT, Kraemer FB, Liu J (2007) Transcriptional activation of hepatic ACSL3 and ACSL5 by oncostatin m reduces hypertriglyceridemia through enhanced beta-oxidation. Arterioscler Thromb Vasc Biol 27:2198–2205. doi:10.1161/ATVBAHA.107.148429
Adamo KB, Dent R, Langefeld CD, Cox M, Williams K, Carrick KM, Stuart JS, Sundseth SS, Harper ME, McPherson R, Tesson F (2007) Peroxisome proliferator-activated receptor gamma 2 and acyl-CoA synthetase 5 polymorphisms influence diet response. Obesity (Silver Spring) 15:1068–1075. doi:10.1038/oby.2007.630
Mourot J, Guy G, Lagarrigue S, Peiniau P, Hermier D (2000) Role of hepatic lipogenesis in the susceptibility to fatty liver in the goose (Anser anser). Comp Biochem Physiol B 126:81–87
Han CC, Wang JW, Pan ZX, Tang H, Xiang SX, Wang J, Li L, Xu F, Wei SH (2011) Effect of liver X receptor activation on the very low density lipoprotein secretion and messenger ribonucleic acid level of related genes in goose primary hepatocytes. Poult Sci 90:402–409. doi:10.3382/ps.2010-00995
Pan Z, Wang J, Tang H, Li L, Lv J, Xia L, Han C, Xu F, He H, Xu H, Kang B (2011) Effects of palmitic acid on lipid metabolism homeostasis and apoptosis in goose primary hepatocytes. Mol Cell Biochem 350:39–46. doi:10.1007/s11010-010-0680-6
Pan Z, Wang J, Kang B, Lu L, Han C, Tang H, Li L, Xu F, Zhou Z, Lv J (2010) Screening and identification of differentially expressed genes in goose hepatocytes exposed to free fatty acid. J Cell Biochem 111:1482–1492. doi:10.1002/jcb.22878
Wang JW, Pan ZX, Lv J, Lu LZ, Li L (2012) Cloning of goose ACSL4 gene and its relationship with lipid accumulation in liver. Chin J Anim Sci 5:6–10
Pan ZX, Lv J, Lu LZ, Wang JW (2010) Cloning of goose ACSL1 gene, tissues expression and the effect of overfeeding on its mRNA level. Acta Veterinaria et Zootechnica Sinica 41:1407–1413
Seglen PO (1973) Preparation of rat liver cells: III. Enzymatic requirements for tissue dispersion. Exp Cell Res 82:391–398
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Ghosh B, Barbosa E, Singh I (1995) Molecular cloning and sequencing of human palmitoyl-CoA ligase and its tissue specific expression. Mol Cell Biochem 151:77–81
Oikawa E, Iijima H, Suzuki T, Sasano H, Sato H, Kamataki A, Nagura H, Kang MJ, Fujino T, Suzuki H, Yamamoto TT (1998) A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J Biochem 124:679–685
King NP, Lee TM, Sawaya MR, Cascio D, Yeates TO (2008) Structures and functional implications of an AMP-binding cystathionine beta-synthase domain protein from a hyperthermophilic archaeon. J Mol Biol 380:181–192. doi:10.1016/j.jmb.2008.04.073
Wu M, Liu H, Chen W, Fujimoto Y, Liu J (2009) Hepatic expression of long-chain acyl-CoA synthetase 3 is upregulated in hyperlipidemic hamsters. Lipids 44:989–998. doi:10.1007/s11745-009-3341-3
Mashek DG, Li LO, Coleman RA (2006) Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res 47:2004–2010. doi:10.1194/jlr.M600150-JLR200
Gassler N, Obermuller N, Keith M, Schirmacher P, Autschbach F (2005) Characterization of metaplastic and heterotopic epithelia in the human gastrointestinal tract by the expression pattern of acyl-CoA synthetase 5. Histol Histopathol 20:409–414
Pan Z, Wang J, Han C, Zhai N, Lv J, Zhou Z, Tang H, Xiang S, Wang J, Li L (2010) Identification of differentially expressed genes between hepatocytes of Landes geese (Anser anser) and Sichuan White geese (Anser cygnoides). Mol Biol Rep 37:4059–4066. doi:10.1007/s11033-010-0065-7
Molette C, Berzaghi P, Zotte AD, Remignon H, Babile R (2001) The use of near-infrared reflectance spectroscopy in the prediction of the chemical composition of goose fatty liver. Poult Sci 80:1625–1629
Brasaemle DL, Dolios G, Shapiro L, Wang R (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem 279:46835–46842. doi:10.1074/jbc.M409340200
Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA (2001) Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem 276:24674–24679. doi:10.1074/jbc.M102036200
Acknowledgments
The National Waterfowl Industrial Technology System (No. CARS-43-6) and the Program for Technology Innovative Research Team of Sichuan Province of China (2011JTD0032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, H., Liu, H.H., Wang, J.W. et al. Molecular cloning of the goose ACSL3 and ACSL5 coding domain sequences and their expression characteristics during goose fatty liver development. Mol Biol Rep 41, 2045–2053 (2014). https://doi.org/10.1007/s11033-014-3053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3053-5