Abstract
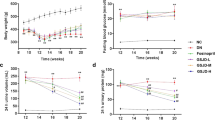
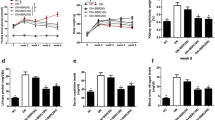
Diabetic nephropathy (DN) is a progressive kidney disease that is caused by injury to glomerulus and glomerular mesangial cells (MCs) proliferation play a critical role in the pathogenesis of DN. The current studies were undertaken to investigate the protective effects and the possible molecular mechanism of berberine on streptozotocin (STZ)-induced DN rats. Male Wistar rats were randomly assigned to normal control and DN groups of comparable age. Three DN groups received 50, 100 and 200 mg/kg of berberine for 8 weeks via daily intragastrically, respectively. The G proteins-adenylyl cyclase (AC)-cAMP signaling pathway and glomerular MCs proliferation were examined in STZ-induced diabetic rat kidney. Enhanced MCs proliferation and remarkable renal injury were concomitant with activation of Gαi and inhibition of Gαs and cAMP in DN model group. Berberine treatment for 8 weeks abolished the above changes by upregulating the expression of Gαs protein and downregulating the expression of Gαi protein, increasing cAMP level, and inhibiting MCs proliferation compared with model group. Taken together, for the first time, these results demonstrated that berberine can relieve renal injury in DN rats through mediating G proteins-AC-cAMP signaling pathway and inhibiting the abnormal proliferation of MCs by increasing cAMP level, suggesting that berberine could be a potential therapeutic agent for the treatment of DN.






Similar content being viewed by others
Abbreviations
- G proteins:
-
Heterotrimeric GTP-binding proteins
- DN:
-
Diabetic nephropathy
- STZ:
-
Streptozotocin
- ECM:
-
Extracellular matrix
- GBM:
-
Glomerular basement membrane
- Camp:
-
Adenosine 3-,5- monophosphate
- AC:
-
Adenylate cyclase
- TGF-β1 :
-
Transforming growth factor-beta1
- FN:
-
Fibronectin
- CTGF:
-
Connective tissue factor
- FBG:
-
Fasting blood glucose
References
Guilbert JJ (2006) The World Health Report 2006: working together for health. Educ Health (Abingdon) 19:385–387
Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4:444–452
Kanwar YS, Sun L, Xie P et al (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6:395–423
Reutens AT, Atkins RC (2011) Epidemiology of diabetic nephropathy. Contrib Nephrol 170:1–7
Kobayashi T, Okada H, Inoue T et al (2006) Tubular expression of connective tissue growth factor correlates with interstitial fibrosis in type 2 diabetic nephropathy. Nephrol Dial Transplant 21:548–549
Guha M, Xu ZG, Tung D et al (2007) Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J 21(12):3355–3368
Umezono T, Toyoda M, Kato M et al (2006) Glomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathy. J Nephrol 19(6):751–757
Zhang Z, Kundu GC, Yuan CJ et al (1997) Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science 276:1408–1412
Striker GE, Peten EP, Carome MA et al (1993) The kidney disease of diabetes mellitus (KDDM): a cell and molecular biology approach. Diabetes Metab Rev 9:37–56
Van Nieuwenhoven FA, Jensen LJ, Flyvbjerg A et al (2005) Imbalance of growth factor signalling in diabetic kidney disease: is connective tissue growth factor (CTGF, CCN2) the perfect intervention point? Nephrol Dial Transplant 20:6–10
Hoffman BB, Sharma K, Ziyadeh FN (1998) Potential role of TGF-βin diabetic nephropathy. Miner Electrolyte Metab 24:190–196
Goldschmeding R, Aten J, Ito Y et al (2000) Connective tissue growth factor: just another factor in renal fibrosis? Nephrol Dial Transplant 15:296–299
Boor P, Sebeková K, Ostendorf T et al (2007) Treatment targets in renal fibrosis. Nephrol Dial Transplant 22:3391–3407
Gupta S, Clarkson MR, Duggan J et al (2000) Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int 58:1389–1399
Wahab NA, Yevdokimova N, Weston BS et al (2001) Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J 359:77–87
Yokoi H, Mukoyama M, Nagae T et al (2004) Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol 15:1430–1440
Giunti S, Barit D, Cooper ME (2006) Diabetic nephropathy: from mechanisms to rational therapies. Minerva Med 97:241–262
Gilman AG (1987) G-proteins: transducers of receptor generated signals. Annu Rev Biochem 56:615–649
Bourne HR, Sanders DA, McCormick F (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125–132
Neer EJ, Heterotrimeric G (1995) Proteins: organizers of transmembrane signals. Cell 80:249–257
Jang IS, Juhnn YS (2001) Adaptation of cAMP signaling system in SH-SY5Y neuroblastoma cells following expression of a constitutively active stimulatory G protein alpha, Q227L Gsalpha. Exp Mol Med 33(1):37–45
Salazar NC, Chen J, Rockman HA (2007) Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta 1768:1006–1018
Yin J, Xing H, Ye J (2008) Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 57:712–717
Yin J, Gao Z, Liu D et al (2008) Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab 294:E148–E156
Singh J, Kakkar P (2009) Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J Ethnopharmacol 123:22–26
Wang Y, Champbell T, Perry B (2011) Hypoglycemic and insulin-sensitizing effects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism 60(2):298–305
Liu WH, Hei ZQ, Nie H et al (2008) Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Med J (Engl) 121:706–712
Liu W, Liu P, Tao S et al (2008) Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch Biochem Biophys 475:128–134
Liu W, Tang F, Deng Y et al (2009) Berberine reduces fibronectin and collagen accumulation in rat glomerular mesangial cells cultured under high glucose condition. Mol Cell Biochem 325:99–105
Liu S, Tang LQ, Chen LM (2004) Study on extraction technology of berberine from Rhizoma coptidis by the method of orthogonal-test optimization. China Pharm 15:18–20
Xu SY, Bian RL, Chen X (2003) Experimental methods in pharmacology. People health publishing, Beijing, p 585
Zhou R, Guo JJ, Yin CM, et al (2003) Clinical observations of Xiaoke Wan in treatment of type 2 diabetic patients (Qi and Yin deficiency zheng). Zhongguo YaoWu Yu Lin Chuang 3:131–132 (Chinese)
Zhang HZ (1999) Clinical observation into Xiaoke Wan in the treatment of 86 cases of type 2 diabetic patients. Zhong Yi Yao Yan Jiu 15:19–21 (Chinese)
Itoh Y, Imamura S, Yamamoto K et al (2002) Changes of endothelin in streptozotocin-induced diabetic rats: effects of an angiotensin converting enzyme inhibitor, enalapril maleate. J Endocrinol 175(1):233–239
Fujiwara Y, Kitamura E, Ochi S et al (1991) Isotopic measurement of glomerular intracapillary volume as a quantitative index for mesangial cell contractility. Contrib Nephrol 95:12–21
Wolf G, Ziyadeh FN (1999) Molecular mechanism of diabetic renal hypertrophy. Kidney Int 56:393–405
Lu SS, Yu YL, Zhu HJ et al (2009) Berberine promotes glucagon-like peptide–1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol 200:159–165
Schena FP (2005) Gesualdo L Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16(Suppl 1):S30–S33
Ichinose K, Kawasaki E, Eguchi K (2007) Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol 27:554–564
Liu Y (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidndy Int 69:213–217
Ruiz-Torres MP, Lopez-Ongil S, Griera M et al (2005) The accumulation of extracellular matrix in the kidney: consequences on cellular function. J Nephrol 18:334–340
Molitch ME, DeFronzo RA, Franz MJ et al (2004) American Diabetes Association. Nephropathy in diabetes. Diabetes Care 27:S79–S83
Chen S, Hong SW, Iglesias-de la Cruz MC et al (2001) The key role of the transforming growth factor-beta system in the pathogenesis of diabetic nephropathy. Renal Fail 23:471–481
Ziyadeh FN, Sharma K, Ericksen M et al (1994) Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor beta. J Clin Invest 93:536–542
Murphy M, Docherty NG, Griffin B et al (2008) IHG-1 amplifies TGF-beta 1 signaling and is increased in renal fibrosis. J Am Soc Nephrol 19:1672–1680
Abdel WN, Mason RM (2004) Connective tissue growth factor and renal diseases: some answers, more questions. Curr Opin Nephrol Hypertens 13:53–58
Mason RM, Wahab NA (2003) Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 14:1358–1373
Liu W, Zhang X, Liu P et al (2010) Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur J Pharmacol 638(1–3):150–155
Kurogi Y (2003) Mesangial cell proliferation inhibitors for the treatment of proliferative glomerular disease. Med Res Rev 23:15–31
Li X, Liu W, Wang Q (2009) Huang, et al. Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol Cell Endocrinol 307:157–162
Hashim S, Liu YY, Wang R et al (2002) Streptozotocin induced diabetes impairs G-protein linked signal transduction in vascular smooth muscle. Mol Cell Biochem 240:57–65
Hashim S, Li Y, Nagakura A, Takeo S et al (2004) Modulation of G-protein expression and adenylyl cyclase signaling by high glucose in vascular smooth muscle. Cardiovasc Res 63:709–718
Zheng XL, Guo J, Wang HY, Malbon CC (1998) Expression of constitutively activated Gialpha2 in vivo ameliorates streptozotocin-induced diabetes. J Biol Chem 273:23649–23651
Li Y, Descorbeth M, Anand-Srivastava MB (2008) Role of oxidative stress in high glucose-induced decreased expression of Giα proteins and adenylyl cyclase signaling in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 294(6):H2845–H2854
Stryer L, Bourne HR (1986) G proteins: a family of signal transducers. Annu Rev Cell Biol 2:391–419
Spiegel AM (1987) Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol 49:1–16
Dumont JE, Jauniaux JC, Roger PP (1989) The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci 14:67–71
Li X, Zarinetchi F, Schrier RW et al (1995) Inhibition of MAP kinase by prostaglandin E2 and forskolin in rat renal mesangial cells. Am J Physiol 269:C986–C991
Hashim S, Li Y, Anand-Srivastava MB (2006) G Protein-linked cell signaling and cardiovascular functions in diabetes/hyperglycemia. Cell Biochem Biophys 44(1):51–64
Tang LQ, Lv F, Liu S et al (2011) Effect of berberine on expression of transforming growth factor-beta1 and type IV collagen proteins in mesangial cells of diabetic rats with nephropathy. Zhongguo Zhong Yao Za Zhi 36:3494–3497 (Chinese)
Acknowledgments
This Project was supported by the National Natural Science Foundation of China (Nos. 81073109, 81102864) and Natural Science Foundation of Anhui Province (China, No. 090413106). We would like to extend our appreciation to Wei Wei and the staff at the School of Pharmacy, Anhui College of Traditional Chinese Medicine for their technical support.
Conflict of interest
There are no competing financial interests in relation to the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, L.Q., Wang, F.L., Zhu, L.N. et al. Berberine ameliorates renal injury by regulating G proteins-AC- cAMP signaling in diabetic rats with nephropathy. Mol Biol Rep 40, 3913–3923 (2013). https://doi.org/10.1007/s11033-012-2468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2468-0