Abstract
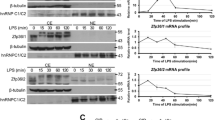
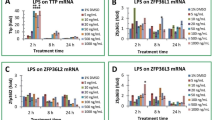
Lipopolysaccharide (LPS) treatment causes the marked changes of gene expression in macrophages. Tristetraprolin (TTP), which is an mRNA-destabilizing protein that negatively regulates the expression of pro-inflammatory mediators, is induced by LPS. To delineate the molecular mechanism of LPS-stimulated TTP expression, several inhibitors blocking different signaling pathways were used initially. We observed that inhibitors of the NF-κB signaling pathway could down-regulate the TTP expression during LPS-induction. Consistently, TTP expression was increased upon recombinant TNFα stimulation which activates NF-κB signaling. The 5′ regulatory region of zfp36 gene spanning from −2 k to +50 was isolated, which contained a putative NF-κB–binding site located in −1859 to −1850. Analysis of luciferase reporter activity driven by a serial 5′-deletion of TTP promoter showed that NF-κB inhibitor-mediated suppression of LPS or TNFα induced activity was through the predicted κB-binding sites. When the NF-κB-binding site was mutated, the TTP promoter decreased its response to the ectopic expression of NF-κB. Physical interaction analysis including oligonucleotides competition, gel shift and chromatin immunoprecipitation assays demonstrated that NF-κB activated by LPS or TNFα bound to the TTP promoter specifically. These results suggested that during LPS stimulation, NF-κB signaling were activated to regulate the transcription of TTP mRNA.





Similar content being viewed by others
Abbreviations
- ARE:
-
AU-rich element
- ChIP:
-
Chromatin-immunoprecipitation
- EMSA:
-
Electrophoretic mobility shift assay
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor-kappa B
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- STAT:
-
Signal transduction and activator of transcription
- TLR:
-
Toll-like receptor
- TNFα:
-
Tumor necrosis factor-alpha
- TTP:
-
Tristetraprolin
- UTR:
-
Untranslated region
References
O’Neill LA (2006) How toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol 18:3–9
Miggin SM, O’Neill LA (2006) New insights into the regulation of TLR signaling. J Leukoc Biol 80:220–226
Zheng S, Brown MC, Taffet SM (1993) Lipopolysaccharide stimulates both nuclear localization of the nuclear factor kappa B 50 kDa subunit and loss of the 105 kDa precursor in RAW264 macrophage-like cells. J Biol Chem 268:17233–17239
Triantafilou M, Triantafilou K (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 23:301–304
Clark AR (2007) Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 275:79–97
Liew FY, Xu D, Brint EK, O’Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5:446–458
Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ (1999) Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol 19:4311–4323
Lai WS, Kennington EA, Blackshear PJ (2003) Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol Cell Biol 23:3798–3812
Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M (2001) AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 107:451–464
Mukherjee D, Gao M, O’Connor JP, Raijmakers R, Pruijn G, Lutz CS, Wilusz J (2002) The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J 21:165–174
Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J (2005) Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell 20:905–915
Lykke-Andersen J, Wagner E (2005) Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev 19:351–361
Carballo E, Lai WS, Blackshear PJ (1998) Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science 281:1001–1005
Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF et al (1996) A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4:445–454
Gomperts M, Pascall JC, Brown KD (1990) Identification of a mRNA rapidly induced in an intestinal epithelial cell line by epidermal growth factor. Biochem Soc Trans 18:568–569
DuBois RN, McLane MW, Ryder K, Lau LF, Nathans D (1990) A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J Biol Chem 265:19185–19191
Leppanen T, Jalonen U, Kankaanranta H, Tuominen R, Moilanen E (2008) Inhibition of protein kinase C beta II downregulates tristetraprolin expression in activated macrophages. Inflamm Res 57:230–240
Leppanen T, Jalonen U, Korhonen R, Tuominen RK, Moilanen E (2010) Inhibition of protein kinase Cdelta reduces tristetraprolin expression by destabilizing its mRNA in activated macrophages. Eur J Pharmacol 628:220–225
Jalonen U, Leppanen T, Kankaanranta H, Moilanen E (2007) Salbutamol increases tristetraprolin expression in macrophages. Life Sci 81:1651–1658
Lai WS, Thompson MJ, Blackshear PJ (1998) Characteristics of the intron involvement in the mitogen-induced expression of Zfp-36. J Biol Chem 273:506–517
Lai WS, Thompson MJ, Taylor GA, Liu Y, Blackshear PJ (1995) Promoter analysis of Zfp-36, the mitogen-inducible gene encoding the zinc finger protein tristetraprolin. J Biol Chem 270:25266–25272
Ogawa K, Chen F, Kim YJ, Chen Y (2003) Transcriptional regulation of tristetraprolin by transforming growth factor-beta in human T cells. J Biol Chem 278:30373–30381
Suzuki K, Nakajima H, Ikeda K, Maezawa Y, Suto A, Takatori H, Saito Y, Iwamoto I (2003) IL-4-Stat6 signaling induces tristetraprolin expression and inhibits TNF-alpha production in mast cells. J Exp Med 198:1717–1727
Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Muller M, Blackshear PJ, Kovarik P (2006) Interferons limit inflammatory responses by induction of tristetraprolin. Blood 107:4790–4797
Chen YL, Huang YL, Lin NY, Chen HC, Chiu WC, Chang CJ (2006) Differential regulation of ARE-mediated TNFalpha and IL-1beta mRNA stability by lipopolysaccharide in RAW264.7 cells. Biochem Biophys Res Commun 346:160–168
Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV (1990) Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med 171:35–47
Hooghe B, Hulpiau P, van Roy F, De Bleser P (2008) ConTra: a promoter alignment analysis tool for identification of transcription factor binding sites across species. Nucleic Acids Res 36:W128–W132
Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T (1997) CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA 94:2927–2932
Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ (1997) Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523–527
Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T (1999) Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol 19:6367–6378
Tchen CR, Brook M, Saklatvala J, Clark AR (2004) The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem 279:32393–32400
Jalonen U, Lahti A, Korhonen R, Kankaanranta H, Moilanen E (2005) Inhibition of tristetraprolin expression by dexamethasone in activated macrophages. Biochem Pharmacol 69:733–740
Palombella VJ, Rando OJ, Goldberg AL, Maniatis T (1994) The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773–785
Deleault KM, Skinner SJ, Brooks SA (2008) Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol 45:13–24
Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR (2006) Post-translational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol 26:2408–2418
King EM, Kaur M, Gong W, Rider CF, Holden NS, Newton R (2009) Regulation of tristetraprolin expression by interleukin-1 beta and dexamethasone in human pulmonary epithelial cells: roles for nuclear factor-kappa B and p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 330:575–585
Shames BD, Selzman CH, Pulido EJ, Meng X, Meldrum DR, McIntyre RC Jr, Harken AH, Banerjee A (1999) LPS-Induced NF-kappaB activation and TNF-alpha release in human monocytes are protein tyrosine kinase dependent and protein kinase C independent. J Surg Res 83:69–74
Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR (2001) Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol 21:6461–6469
Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M (2006) Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol 26:2399–2407
Brooks SA, Connolly JE, Rigby WF (2004) The role of mRNA turnover in the regulation of tristetraprolin expression: evidence for an extracellular signal-regulated kinase-specific, AU-rich element-dependent, autoregulatory pathway. J Immunol 172:7263–7271
Lin NY, Lin CT, Chen YL, Chang CJ (2007) Regulation of tristetraprolin during differentiation of 3T3-L1 preadipocytes. FEBS J 274(3):867–878
Schichl YM, Resch U, Hofer-Warbinek R, de Martin R (2009) Tristetraprolin impairs NF-kappaB/p65 nuclear translocation. J Biol Chem 284:29571–29581
Liang J, Lei T, Song Y, Yanes N, Qi Y, Fu M (2009) RNA-destabilizing factor tristetraprolin negatively regulates NF-kappaB signaling. J Biol Chem 284:29383–29390
Kracht M, Saklatvala J (2002) Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine 20:91–106
Clark AR, Dean JL, Saklatvala J (2003) Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett 546:37–44
Acknowledgments
We thank Dr. Bertrant C.M. Tan for the critical reading the manuscript; Ms Yi-Li Liu and I-Ching Huang for technical support on DNA sequencing (ABI 3730) supported in part by Department of Medical Research in NTUH. This study was supported by Academia Sinica and a grant from National Taiwan University (96R0066-33 to M.-S. Chang) and a grant from the National Science Council (NSC97-2311-B-001-019-MY3 to C.-J. Chang).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, YL., Jiang, YW., Su, YL. et al. Transcriptional regulation of tristetraprolin by NF-κB signaling in LPS-stimulated macrophages. Mol Biol Rep 40, 2867–2877 (2013). https://doi.org/10.1007/s11033-012-2302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2302-8