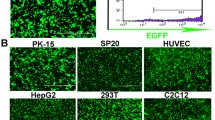
Abstract
Enterotoxigenic Escherichia coli F18 is a major pathogen that causes postweaning diarrhoea and edema disease in piglets. The alpha(1,2)-fucosyltransferase (FUT1) gene has been identified as an ideal candidate gene for controlling the expression of the receptor for ECF18 bacteria. Therefore, the use of RNA interference (RNAi) to study the function of the FUT1 gene and to produce FUT1 knockdown transgenic pig would be highly beneficial. We developed an effective strategy for the expression of multiple small hairpin RNA simultaneously using multiple RNA polymerase III (hU6, hH1, mU6 and h7SK) promoters in a single vector to knockdown the FUT1 gene. Stable FUT1 knockdown transgenic fibroblast lines were generated by transfecting porcine fetal fibroblasts with the constructed vectors. Real-time RT-PCR indicated that the mRNA level of FUT1 in the transgenic fibroblast lines was significantly lower than that in the control, as much as 29 %. Finally, we successfully obtained transgenic SCNT porcine embryos. Overall, the results demonstrated that this vector-based RNAi expression system is an efficient approach to knockdown FUT1 gene expression in porcine fetal fibroblast cells, which could thereby provide donor cells for somatic cell nuclear cloning and the potential production of a marker-free transgenic pig resistant to F18 related diseases. Furthermore, it also provides strong evidence that this approach could be useful both in the production of transgenic livestock resistant to disease, and in the development of effective strategies for the suppression of gene expression in clinical gene therapy.







Similar content being viewed by others
References
Hampson DJ (1994) Postweaning Escherichia coli diarrhoea in pigs. In: Gyles CL (ed) Escherichia coli in domestic animals and humans. CAB International, Oxford, pp 171–191
Verdonck F, Cox E, Ampe B et al (2003) Open status of pig-breeding farms is associated with slightly higher seroprevalence of F18+ Escherichia coli in northern Belgium. Prev Vet Med 60(2):133–141
Benin AM, Ducher-Suchaux MF (1991) Relationship between virulence and adherence of various enterotoxigenic Escherichia coli: strains to isolated intestinal epithelial cells from Chinese Meishan and European large white pigs. Am J Vet Res 52:45–49
Bertschinger HU, Stamm M, Vögeli P (1993) Inheritance of resistance to oedema disease in the pig: experiments with an Escherichia coli strain expressing fimbriae 107. Vet Microbiol. 35(1–2):79–89
Meijerink E, Fries R, Vogeli P et al (1997) Two α (1,2) fucosyltransferase genes on porcine chromosome 6q11 are closely linked to the blood group inhibitor (S) and Escherichia coli F18 receptor (ECF18R) Loci. Mamm Genome 8(10):736–741
Meijerink E, Neuenschwander S, Fries R et al (2000) A DNA polymorphism influencing alpha(1,2)fucosyltransferase activity of the pig FUT1 enzyme determines susceptibility of small intestinal epithelium to Escherichia coli F18. Immunogenetics 52:129–136
Elbashir SM, Harborth J, Lendeckel W et al (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553
Sui G, Soohoo C, Affare B et al (2002) A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 99:5515–5520
Henry SD, van der WP, Metselaar HJ et al. (2006) Simultaneous targeting of HCV replication and viral binding with a single lentiviral vector containing multiple RNA interference expression cassettes. Mol Ther 14: 485–493
Hung CF, Cheng TL, Wu RH et al (2006) A novel bidirectional expression system for simultaneous expression of both the protein-coding genes and short hairpin RNAs in mammalian cells. Biochem Biophys Res Commun 339:1035–1042
Jazag A, Kanai F, Ijichi H et al (2005) Single small-interfering RNA expression vector for silencing multiple transforming growth factor-beta pathway components. Nucleic Acids Res 33:e131
Schuck S, Manninen A, Honsho M et al (2004) Generation of single and double knockdowns in polarized epithelial cells by retrovirus-mediated RNA interference. Proc Natl Acad Sci USA 101:4912–4917
Veronique S, Kaatje S, Evelien N et al (2006) Multiple gene knock-down by a single lentiviral vector expressing an array of short hairpin RNAs. Electron J Biotechnol 9(5):572–579
Sun D, Melegari M, Sridhar S et al (2006) Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. Biotechniques 41:59–63
Gou D, Weng T, Wang Y et al (2007) A novel approach for the construction of multiple shRNA expression vectors. J Gene Med 9(9):751–763
Motegi Y, Katayama K, Sakurai F et al (2011) An effective gene-knockdown using multiple shRNA-expressing adenovirus vectors. J Control Release 153(2):149–153
Junn HJ, Kim JY, Seol DW (2010) Effective knockdown of multiple target genes by expressing the single transcript harbouring multi-cistronic shRNAs. Biochem Biophys Res Commun 396(4):861–865.
Liu HS, Jan MS, Chou CK et al (1999) Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260(3):712–717
Agbulut O, Coirault C, Niederländer N et al (2006) GFP expression in muscle cells impairs actin-myosin interactions: implications for cell therapy. Nat Methods 3(5):331
Krestel HE, Mihaljevic AL, Hoffman DA et al (2004) Neuronal co-expression of EGFP and beta-galactosidase in mice causes neuropathology and premature death. Neurobiol Dis 17(2):310–318
Dixit R, Cyr R (2003) Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J 36(2):280–290
Huang WY, Aramburu J, Douglas PS et al (2000) Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med 6(5):482–483
Grimm D, Streetz KL, Jopling CL et al. (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441(7092):537–541.
Baens M, Noels H, Broeckx V et al (2006) The dark side of EGFP: defective polyubiquitination 1:e54
Torbett BE (2002) Reporter genes: too much of a good thing? J Gene Med 4(5):478–479
Daigle DM, McKay GA, Thompson PR et al (1999) Aminoglycoside antibiotic phosphotransferases are also serine protein kinases. Chem Biol 6(1):11–18
Miki B, Abdeen A, Manabe Y et al (2009) Selectable marker genes and unintended changes to the plant transcriptome. Plant Biotechnol J 7(3):211–218
ter Brake O, ‘t Hooft K, Liu YP et al (2008) Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther 16(3):557–564
Acknowledgments
We are indebted to professor Zhengxing Lian for providing 3s-loxP-GFP-TLR4, we thanks Mr. Qi Wang and Hui Feng for providing experimental supplies. This work was supported by the National Transgenic Breeding Program of China (2009ZX08006-004B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Jw., Zhang, Y., Zhang, Yl. et al. Construction of multiple shRNAs expression vector that inhibits FUT1 gene expression and production of the transgenic SCNT embryos in vitro. Mol Biol Rep 40, 2243–2252 (2013). https://doi.org/10.1007/s11033-012-2287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2287-3