Abstract
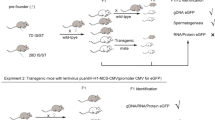
The objective of this study was to explore the possibility of obtaining stable transgenic animals by intratesticular injection. The recombinant vector pEGFP-H-FABP expressing the goat heart-type fatty acid binding protein and green fluorescent protein was mixed with liposome complexes and randomly injected into the testes of mice. Testicular section, fluorescence, and DNA detection assays of mouse sperm were performed to determine the integration of foreign DNA. The results showed that foreign DNA was successfully expressed in the treated mice. Furthermore, the expression and function of the foreign gene were analyzed in F1 generation and F2 generation mice at different levels, with the positive rates of foreign gene transfer into the F1 and F2 generations being 4.0 and 30.23 %, respectively. These results strongly support testicular injection as an effective method of producing transgenic animals and indicate that foreign genes can be stably passed on to the offspring. This research has theoretical and practical implications for the improvement in the quality of laboratory animals and for gene therapy.










Similar content being viewed by others
References
Smith KR (2004) Gene therapy: the potential applicability of gene transfer technology to the human germline. Int J Med Sci 1:76–91
Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH (1980) Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A 77:7380–7384
Arezzo F (1989) Sea urchin sperm as a vector of foreign genetic information. Cell Biol Int Reports 13:391–404
Lavitrano M, Camaioni A, Fazio VM, Dolci S, Farace MG, Spadafora C (1989) Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell 57:717–723
Sperandio S, Lulli V, Bacci M, Forni M, Maione B, Spadafora C, Lavitrano M (1996) Sperm-mediated DNA transfer in bovine and swine species. Animal Biotechnol 7:59–77
Gandolfi F, Lavitrano M, Camaioni A, Spadafora C, Siracusa G, Lauria A (1989) The use of sperm-mediated gene transfer for the generation of transgenic pigs. J Reprod Fertil 4:10
Wu J, Liu M, Li W, Huang J, Shi H, Guo Z (2001) Study for production of transgenic sheep by sperm mediated exogenous DNA method. Herbiv Animals S2:186–202
Farre L, Rigau T, Mogas T, García-Rocha M, Canal M, Gomez-Foix A, Rodríguez-Gil J (1999) Adenovirus-mediated introduction of DNA into pig sperm and offspring. Mol Reprod Dev 53:149–158
Sato M (1999) Testis-mediated gene transfer (TMGT) in mice: successful transmission of introduced DNA from F0 to F2 generations. Transgenics 3:11–22
Zhao J, Liu B, Ren WZ, Zhang SF, Yu L, Li YP, Qiao GL, Hu RL, Yin Z (2003) Production of transgenic mice by in vivo spermatogonia-mediated gene transfer. Shi yan sheng wu xue bao 36:197
Shen X, Qiao G, Zhang L, Jiang P, Huang H, Yao K (2002) Construction of transgenic mice carrying enhanced green fluorescent protein gene by seminiferous tubule microinjection. Di 1 jun yi da xue xue bao=Academic J First Med College PLA 22:50
Yonezawa T, Furuhata Y, Hirabayashi K, Suzuki M, Takahashi M, Nishihara M (2001) Detection of transgene in progeny at different developmental stages following testis-mediated gene transfer. Mol Reprod Dev 60:196–201
Gao H, Cao Y, Li S, Ren Y, Li Q (2003) Research on spermatozoa-mediated gene transfer to product transgenic animals. Hereditas 25:283
Li F, Wei H, Sun X, Zhao Y (2005) Transfection of pEGFP-N1 gene into goat spermatozoa in vivo and its expression in early embryos after transfection. Acta Lab Anim Sci Sin 13:110–113
Storch J, Thumser AE (2010) Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem 285:32679–32683
Erol E, Cline GW, Kim JK, Taegtmeyer H, Binas B (2004) Nonacute effects of H-FABP deficiency on skeletal muscle glucose uptake in vitro. Am J Physiol Endocrinol Metab 287:E977–E982
Tyra M, Ropka-Molik K, Eckert R, Piórkowska K, Oczkowicz M (2011) H-FABP and LEPR gene expression profile in skeletal muscles and liver during ontogenesis in various breeds of pigs. Domest Anim Endocrinol 40:147–154
Chang LL, Xiao YS, He M, Yu CP (2009) Effects of H-FABP gene polymorphisms and nutritional factors on pork quality. Hereditas (Beijing) 31:713–718
Bachiller D, Schellander K, Peli J, Rüther U (1991) Liposome-mediated DNA uptake by sperm cells. Mol Reprod Dev 30:194–200
Lavitrano M, Forni M, Bacci ML, Di SC, Varzi V, Wang H, Seren E (2003) Sperm mediated gene transfer in pig: selection of donor boars and optimization of DNA uptake. Mol Reprod Dev 64:284–291
Nakanishi A, Iritani A (1993) Gene transfer in the chicken by sperm-mediated methods. Mol Reprod Dev 36:258–261
Xie C, Yu MJ, Hui N, Wang LB, Li WJ, Zhao YJ (2010) Testis injection of exogenous DNA results in expression in mouse sperm. Chin J Zool 2:151–157
Kim JH, Jung-Ha HS, Lee HTL, Chung KS (1997) Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol Reprod Dev 46:515–526
Acknowledgments
The project supported by national new varieties of GMO cultivation (No. 2011ZX08008-003; No. 2009ZX08008-003B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hao Chen and Yanhui Yin contributed equally to this article.
Rights and permissions
About this article
Cite this article
Chen, H., Yin, Y., Zhang, Y. et al. Production of transgenic mice expressing the goat H-FABP gene by intratesticular injection. Mol Biol Rep 40, 2215–2222 (2013). https://doi.org/10.1007/s11033-012-2283-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2283-7