Abstract
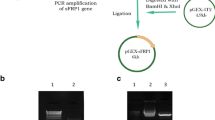
Receptor-interacting protein 2 (RIP2) is a member of the receptor interacting protein (RIP) family and plays an important role in the innate and adaptive immune responses. Overexpression of RIP2 mediates divergent signaling pathways including NF-κB activation and cell death. To further investigate the biological activity of RIP2 in vitro, a large amount of purified protein is required. For this purpose, the full length of RIP2 was cloned from human Ramos (human Burkitt lymphoma) tumor cells and inserted in a prokaryotic expression vector pET22b, and then the recombinant plasmid was transformed into E. coli BL21 (DE3) competent cells. The expression of RIP2 was induced with IPTG. SDS-PAGE analysis showed that recombinant human RIP2 (rhRIP2) was mainly expressed as soluble fraction in the supernatant of the cell lysate. The recombinant protein was subsequently purified by His Trap FF crude to a purity of 90 %. MTT assay of the purified rhRIP2 showed its functional diversity in different cell lines, a specific inhibitory effect on MCF7 cells, but a promotion on the proliferation of Ramos cells. Furthermore, we identified that rhRIP2 could suppress activation of canonical NF-κB in MCF7 cells and activate non-canonical NF-κB signaling in Ramos cells, these data suggested that RIP2 participates in different signaling pathways contributing to its specific effects in vitro. Our results provided new clues to further explore the regulation mechanisms of RIP2 in tumorigenesis.






Similar content being viewed by others
References
McCarthy JV, Ni J, Dixit VM (1998) RIP2 is a novel NF-kappaB activating and cell death-inducing kinase. J Biol Chem 273:16968–16975
Inohara N, DelPeso L, Koseki T, Chen S, Nunez G (1998) RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis. J Biol Chem 273:12296–12300
Thome M, Hofmann K, Burns K, Martinon F, Bodmer JL, Mattmann C, Tschopp J (1998) Identification of CARDIAK, a RIPlike kinase that associates with caspase-1. Curr Biol 8:885–888
Stanger BZ, Leder P, Lee TH, Kim E, Seed B (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81:513–523
Meylan E, Martinon F, Thome M, Gschwendt M, Tschopp J (2002) RIP4 (DIK/PKK), a novel member of the RIP kinase family, activates NF-kappa B and is processed during apoptosis. EMBO Rep 3:1201–1208
Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, Nolan GP, Payan DG, Luo Y (1999) Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol 9:539–542
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM (1999) RIP3, a novel apoptosis-inducing kinase. J Biol Chem 274:16871–16875
Zha J, Zhou Q, Xu LG, Chen D, Li L, Zhai Z, Shu HB (2004) RIP5 is a RIP-homologous inducer of cell death. Biochem Biophys Res Commun 319:298–303
Meylan E, Tschopp J (2005) The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci 30:151–159
Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA (2002) RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194–199
Ehlers S, Mueck T, Adams S, Landuzzi L, Lollini PL, Munz B (2008) RIP2 regulates growth and differentiation of normal myoblasts and of rhabdomyosarcoma cells. Eur J Cell Biol 87:163–172
Adams S, Valchanova RS, Munz B (2010) RIP2: a novel player in the regulation of keratinocyte proliferation and cutaneous wound repair? Exp Cell Res 316:728–736
Hasegawa M, Fujimoto Y, Lucas P, Nakano H, Fukase K, Nu′n˜ez G, Inohara N (2008) A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J 27:373–383
Wada K, Maeda K, Tajima K, Kato T, Kobata T, Yamakawa M (2009) Expression of BAFF-R and TACI in reactive lymphoid tissues and B-cell lymphomas. Histopathology 54(2):221–232
Pham LV, Fu L, Tamayo AT, Bueso-Ramos C, Drakos E, Vega F, Medeiros LJ, Ford RJ (2011) Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-κB-inducing kinase while activating both canonical and alternative nuclear factor-κB pathways. Blood 117(1):200–210
Studier FW, MoVatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr (1997) Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 17:3629–3639
Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE (1997) Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Investig 100:2952–2960
Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE (2007) Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol 9(4):470–478
Gavert N, Ben-Ze’ev A (2008) Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med 14:199–209
Kim DS, Park KS, Kim SY (2009) Silencing of TGase 2 sensitizes breast cancer cells to apoptosis by regulation of survival factors. Front Biosci 14:2514–2521
Jinsong Y, Ming S, Aiping Z, Chengyu L, Wei D, Zhaoxia W (2011) Adenovirus-mediated siRNA targeting Bcl-xL inhibits proliferation, reduces invasion and enhances radiosensitivity of human colorectal cancer cells. World J Surg Oncol 9:117
Watanabe J, Kushihata F, Honda K, Mominoki K, Matsuda S, Kobayashi N (2002) Bcl-xL overexpression in human hepatocellular carcinoma. Int J Oncol 21:515–519
Castilla C, Congregado B, Chinchón D, Torrubia FJ, Japón MA, Sáez C (2006) Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology 147:4960–4967
Wang ZX, Yang JS, Pan X, Wang JR, Li J, Yin YM, De W (2010) Functional and biological analysis of Bcl-xL expression in human osteosarcoma. Bone 47:445–454
Hildebrand JM, Luo Z, Manske MK, Price-Troska T, Ziesmer SC, Lin W, Hostager BS, Slager SL, Witzig TE, Ansell SM, Cerhan JR, Bishop GA, Novak AJ (2010) A BAFF-R mutation associated with non-Hodgkin lymphoma alters TRAF recruitment and reveals new insights into BAFF-R signaling. J Exp Med 207(12):2569–2579
Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML (2010) B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev 237(1):205–225
Onda K, Iijima K, Katagiri YU, Okita H, Saito M, Shimizu T, Kiyokawa N (2010) Differential effects of BAFF on B cell precursor acute lymphoblastic leukemia and Burkitt lymphoma. Int J Hematol 91(5):808–819
Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ (2006) Constitutive NF-κB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood 107:4540–4548
Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F (2000) BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 192:1453–1466
Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-kiang S (2000) Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 192:953–964
Rolink AG, Tschopp J, Schneider P, Melchers F (2002) BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol 14:266–275
Huang X, Di Liberto M, Cunningham AF, Kang L, Cheng S, Ely S, Liou HC, Maclennan IC, Chen-Kiang S (2004) Homeostatic cell-cycle control by BLyS: induction of cell-cycle entry but not G1/S transition in opposition to p18 INK4c and p27 Kip1. Proc Natl Acad Sci USA 101: 17789–17794
Acknowledgments
This research was supported by Grants from the Major State Basic Research Development Program of China (973 Program) (No. 2011CB503803) and National Natural Science Foundation of China (No. 30873030, No. 81071928 and No. 81001175).
Author information
Authors and Affiliations
Corresponding authors
Additional information
D. Xu and S. Hang are co-corresponding authors.
Rights and permissions
About this article
Cite this article
Cai, X., Wang, M., Kong, H. et al. Prokaryotic expression, purification and functional characterization of recombinant human RIP2. Mol Biol Rep 40, 59–65 (2013). https://doi.org/10.1007/s11033-012-1995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1995-z