Abstract
The 677C>T polymorphism within methylenetetrahydrofolate reductase (MTHFR) gene is related to an elevated level of homocysteine. Thus it may be considered as a genetic risk factor in ischemic stroke. Apparently studies of this type of polymorphism in childhood stroke have shown conflicting results. We performed meta-analysis of all the data that are available in relation with MTHFR polymorphism and the risk of ischemic stroke in children. We searched PubMed (last search dated December 2010) using “MTHFR polymorphism”, “ischemic stroke” “child”, “children”, “pediatric stroke” as keywords and reference lists of studies and reviews on the topic. Finally, 15 case–control studies corresponded to the inclusion criteria for meta-analysis. These studies involved the total number of 822 children and adolescents after ischemic stroke and 1,552 control subjects. Fixed or random effects models were used depending on the heterogeneity between the studies. The association between ischemic stroke and 677C>T polymorphism within MTHFR gene was observed in three of the studies. The pooled analysis showed that TT genotype of MTHFR gene is more common in stroke patients than in controls (p = 0.0402, odds ratio = 1.57, 95 % confidence interval 1.02–2.41). The Egger’s test did not reveal presence of a publication bias. The results based on a sizeable group of cases and controls have proved that the 677C>T polymorphism in MTHFR gene is associated with the development of ischemic stroke in children.
Similar content being viewed by others
Introduction
Ischemic stroke is a relatively rare disease in children. The incidence of the disease comes to about 3 children per 100,000 children per year [1, 2]. Data from family studies and twin studies suggest that genetic risk factors play an important role in the pathogenesis of ischemic stroke [3, 4]. Stroke is a multifactorial disease that may result from interactions between many risk factors: genetic and non-genetic, as well as environmental ones. Rates of recurrence, mortality and neurological deficits in pediatric stroke patients are significant and concern more than two thirds of the affected children [5]. Neurological deficits and post-stroke disability place a heavy burden on the societies.
Case–control approaches are currently being widely used to determine risk factors in ischemic stroke in adults and children. The 677C>T polymorphism in methylenetetrahydrofolate reductase (MTHFR) gene is one of the most extensively investigated candidate polymorphisms. MTHFR catalyses the reduction of 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate, which is the main form of folate in plasma and a carbon donor for the remethylation of homocysteine to methionine [6]. A single base pair (677C>T) transition in the MTHFR gene influences enzyme thermolability, its decreased activity and, in turn, the elevated level of homocysteine. Thus the MTHFR polymorphism is suggested to be a genetic risk factor in cerebrovascular diseases, including ischemic stroke [7].
As mentioned earlier, studies of this variant in childhood stroke have revealed conflicting results. Some of the reports have showed the relation between MTHFR 677C>T polymorphism and stroke in children [8–10], while other studies have not indicated such correlations [11, 12]. This may be due to the heterogenic character of this type of stroke as different groups of patients may have various sets of genetic factors which predispose to the disease. Some alleles of candidate genes may be strongly associated with the disease in one population, whereas in another this correlation may be weak due to the presence of other genetic factors or specific interactions between genetic and non-genetic factors.
Meta-analysis is a method that enables pooling data from smaller inconclusive studies and yields it with a greater statistical power and allows one to quantify genetic risks more precisely.
We performed meta-analysis of all the data that has been published so far in relation to the risk of ischemic stroke in children with the MTHFR 677C>T polymorphism. We aimed to describe the association between TT genotype of MTHFR gene and the risk of childhood ischemic stroke by meta-analysis.
Methods
Data acquisition
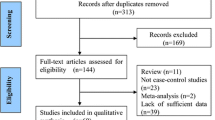
In the first place we identified all articles published before December 2010 on the MTHFR 677C>T polymorphism and its association with ischemic stroke in pediatric patients. Two independent investigators searched the literature using PubMed (last search December 2010). All the references cited in the found studies were also reviewed in order to find other published articles that had been indexed by PubMed. The language was limited to English. The key words used for this search were: “MTHFR polymorphism”, “ischemic stroke”, “child”, “children”, “pediatric stroke”. Abstracts were not included in the search. The inclusion criteria were as follows: (a) a case–control study, (b) study population or at least a subgroup comprising children (between birth and puberty, till 12 ages) and adolescents (between the ages of 13 and 19); in some studies this population included neonates and perinatal stroke patients (c) confirmed ischemic stroke with magnetic resonance imaging (MRI) or computer tomography (CT). A given study was excluded from this meta-analysis when: (a) genotype or allele frequencies were not reported, (b) study design was other than case–control, (c) the associations between MTHFR polymorphism and stroke in adults were investigated, (d) outcome definition was other than ischemic stroke. We attempted to contact the authors of the found reports to get additional data. In case of lack of relevant data or response from the author of a particular work, the study was excluded. Unavailability of data or failure to answer (after repeated attempts) resulted in the exclusion of the study. Of the found publications, 15 studies published during the period from 1998 to 2009 satisfy the inclusion criteria [8–22], including our previously published data [8]. Three of the articles found in PubMed were not included in the meta-analysis because of the overlap of the patients group in other publications [23–25]. We also excluded one publication because the age of the analyzed population ranged from 7 to 36 years [26]. No subgroup containing pediatric population was distinguished so we decided not to the analyze entire data. Data from 15 published studies were included in meta-analysis, comprising in total 822 cases and 1,552 controls.
The meta-analysis was performed in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [27].
Statistical analyses
The data were analyzed by means of the MIX 1.7 software [28, 29]. To determine the strength between genetic polymorphism and childhood ischemic stroke pooled odds ratio (OR) was calculated together with 95 % confidence intervals (CI). Heterogeneity between the studies was evaluated using the Dersimonian and Laird’s Q test. If the p value was less than 0.05, the heterogeneity was considered statistically significant. The I2 metric describes the percentage of the observed between-study variability due to heterogeneity rather than sampling error and was used to quantify heterogeneity. The I2 values ranged between 0 and 100 %, with higher values indicating a greater degree of heterogeneity. In case of significant heterogeneity observed between the studies, the pooled OR was estimated using a random effects model, otherwise a fixed effects model was used. Publication bias was examined with the Egger’s regression asymmetry test.
Results
Characteristics of the included studies
Thirteen of the analyzed studies involved less than one hundred pediatric stroke patients. The largest group was analyzed by Nowak-Göttl et al. [19], the least group of cases was studied by Morita et al. [13]. The highest distributions of the TT genotype in the patients’ groups were observed by Cardo et al. [18]—29 %, Morita et al. [13]—26 % and Nowak-Göttl et al. [19]—24 %. Frequency of the TT homozygotes among controls was the highest in the studies of Rook et al. [11]—19 %, Komitopoulou et al. [17]—17 % and Kenet et al. [10]—15 %. Additionally, in one publication analysis of the families (both of the parents and their affected offspring) was performed except for a case–control study [8].
Characteristics of individuals included to this meta-analysis is shown in Table 1.
A total number of 2,374 individuals were involved in this meta-analysis, including 822 children and adolescents after ischemic stroke and 1,552 control subjects. In all of the 15 studies, different racial populations were analyzed, however Caucasians were the most common.
The association between 677C>T polymorphism within MTHFR gene was observed in three of the analyzed studies [8, 9, 19]. The strongest relationship between MTHFR polymorphism and childhood ischemic stroke was observed by Biswas et al. [9] although the result concerned synergistic effect between HPA-1 and MTHFR polymorphisms. The other of the included studies showed no relations between MTHFR polymorphism and ischemic stroke in childhood [10–18, 20–22] (Table 1).
MTHFR polymorphism and childhood stroke
Summary frequencies of MTHFR genotypes and alleles from all the studies included in the meta-analysis are presented in Table 1.
There was significant heterogeneity between the analyzed studies observed for the following analyses: I2 = 47.4 %, p = 0.025 in case of TT versus CC + CT analysis and therefore random effects model with Dersimonian Laird test was used to analyze this combination.
The pooled analysis showed that TT genotype of MTHFR gene is more common in stroke patients than in controls when compared to CC + CT genotypes (p = 0.0402, OR = 1.57, 95 % CI 1.02–2.41) (Fig. 1). For the MTHFR gene polymorphism, the Egger’s test did not reveal presence of a publication bias (z = 0.22, p value (two-tailed) = 0.825).
Meta-analysis of association between TT genotype of MTHFR gene and pediatric ischemic stroke. OR and 95 % CI of the TT genotype versus CC + CT were calculated from data presented in individual studies. A random effects model with the method of Dersimonian Laird was used to calculate pooled weighted OR. One study was excluded from the calculation because of the lack of TT genotype, both in cases and controls [14]
Additionally, in 11 of the studies we calculated association between carriers of MTHFR T allele (subjects with CT + TT genotypes) and wild-type homozygous. The pooled analysis showed that carriers of T allele are more common in stroke patients than in controls when compared to individuals with CC genotypes (p = 0.014, OR = 1.52, 95 % CI 1.09–2.12) (Fig. 2).
Meta-analysis of association between carriers of T allele (individuals with CT + TT genotypes) of MTHFR gene and pediatric ischemic stroke. OR and 95 % CI of the CT + TT genotypes versus CC were calculated from data presented in individual studies. A random effects model with the method of Dersimonian Laird was used to calculate pooled weighted OR. Four of the studies were excluded from the calculation due to lack of accurate data of CT genotype, both in cases and controls [10, 19, 21, 22]
Sensitivity analysis
According to our calculation, distribution of the MTHFR genotypes in controls differed from Hardy–Weinberg equilibrium (HWE) in one of the analyzed studies, what suggested possible genotyping errors or population bias (χ2 = 4.033, p = 0.045), although the result was close to the bound of significance. Exclusion of this study did not alter the results, thus suggested stability of the present meta-analysis.
Discussion
In the present study we investigate association between 677C>T polymorphism in MTHFR gene and ischemic stroke in children. Data concerning relations between genetic risk factors and pediatric stroke are scarce in comparison to adult stroke analyses. In some pediatric patients the etiology of stroke is not fully understood, however, we may, as it was previously suggested, attribute it to the significant role a genetic factor may play in the development of ischemic stroke [4]. The MTHFR 677C>T polymorphism may be related to higher levels of homocysteine and in consequence to a higher stroke risk [6, 7, 30]. According to Fowler [31] the homozygous variant of MTHFR gene is present in 5–18 % of the population. Hyperhomocysteinemia may consequently lead to endothelial dysfunction of arteries, an early step in the development of atherosclerosis [7]. Homocysteine also stimulates elevation of superoxide anion, platelet aggregation and decreases nitric oxide bioavailability [32]. Thus elevated homocysteine is an established risk factor in venous and arterial thrombosis although there are some discrepancies on association between MTHFR polymorphism and the increased level of HCys. Some studies indicate such relations with Hcys levels elevated over 25 % in TT individuals compared to subjects with CC genotype [6]. It was also demonstrated, in a general population, that individuals with TT genotype and a low plasma folate level or a low folate intake, had higher total plasma homocysteine concentrations than subjects with CT and CC genotypes [33]. On the other hand, Alsayouf et al. [34] found no relation between TT genotype and homocysteine concentration and observed minimal role of 677C>T polymorphism and stroke in children. According to Unal et al. [35] MTHFR polymorphism analyzed alone, without level of homocysteine, had no important role in pediatric ischemic stroke. Among analyzed studies, the association between 677C>T polymorphism within MTHFR gene was observed in three reports [8, 9, 19], the rest of the studied data did not confirm the relation [10–18, 20–22]. These discrepancies may be mainly due to a low number of patients in the analyzed groups.
Under these controversial results, we decided to perform meta-analysis based on a larger group of cases and controls than in single studies to estimate whether there is any relation between TT homozygous state and pediatric ischemic stroke.
Results obtained from the analyzed group of cases and controls confirmed that TT genotype of 677C>T polymorphism as well as carrier-state of T allele are associated with the development of childhood ischemic stroke. Caucasians were the most common race among the analyzed subjects. Racial-ethnic differences in distribution of the 677C>T polymorphism in MTHFR gene have already been described [36, 37]. Klerk et al. [38] demonstrated that the association between MTHFR 677C>T polymorphism and coronary heart disease was weaker in European populations, than that observed in Asian populations, which might be partially attributable to the difference in folate intake between the two ethnic groups.
Earlier studies on MTHFR polymorphism in different populations yielded equivocal results on associations between MTHFR 677C>T polymorphism and stroke, both in adults and children. McColgan and Sharma [39] found no relation between MTHFR polymorphism and carotid dissection, a common cause of stroke in young adults accounting for 20 % of strokes. Meta-analysis of three candidate genes: MTHFR, ACE and APOE in Asian population, revealed a significant association of stroke with the MTHFR 677C>T polymorphism and APOE epsilon 4 alleles, in contrast to ACE gene insertion/deletion polymorphism [40]. In another study based on about 7,000 stroke patients, a gradual increase in the ischemic stroke risk with increasing MTHFR 677T allele dose was observed, which suggests that MTHFR polymorphism may play a significant role in genetic susceptibility to stroke [41]. Meta-analysis of several studies analyzing correlation between MTHFR polymorphism and levels of Hcys and stroke suggested that MTHFR TT genotype may have a small role in determining susceptibility to ischemic stoke [42]. Kelly et al. [42] have also found correlation between mild-to-moderate hyperhomocysteinemia and stroke in the group of about 2,500 stroke patients. On the other hand Wu and Tsongalis [43] confirmed the association between the TT homozygous variant of MTHFR gene and coronary artery disease (CAD) but denied its connections with the stroke.
Previous data concerning the role of MTHFR polymorphism and CAD also confirmed the fact that individuals with the TT genotype had a significantly higher risk of CAD, particularly in the setting of low folate status [38]. The results of meta-analysis carried out by Klerk et al. [38] proved that impaired folate metabolism, resulting in high homocysteine levels, is related to an increased risk of CAD. In another group of patients with CAD it was found that carrier-state of T allele of MTHFR polymorphism, together with other polymorphic variants of candidate genes increased the risk of the disease, especially in women [44]. Another data of MTHFR polymorphism on venous thrombosis demonstrated that MTHFR TT genotype was associated with a 20 % higher risk of the venous thrombosis development than in case of to the CC genotype [45].
The present study has some limitations: the largest one is the variability between populations of the prevalence of MTHFR TT genotype in controls which ranges from 0 % [9, 14] to 19 % [11]. Such differences may be due to a different number of individuals recruited to the analyzed studies. Previously, Franco et al. [37] showed that 677C>T polymorphism in MTHFR gene has a significantly heterogeneous distribution among different ethnic groups and this may explain geographical or racial differences that are linked to the risk of cerebrovascular diseases. Another limitation of our meta-analysis is the fact that in some of the analyzed data included neonates with stroke which also became inclusion criterion in the present meta-analysis, although some publications differentiate neonatal stroke from childhood stroke [46].
To the best of our knowledge, this is the first meta-analysis concerning relationship between MTHFR polymorphism and the risk of pediatric stroke, including data from Polish studies. What is more, the present meta-analysis encompasses over eight hundred children after ischemic stroke and provides more reliable evidence on the role of the 677TT genotype in the pathogenesis of pediatric stroke than scarce data based on small group of patients.
References
Broderick J, Talbot GT, Prenger E et al (1993) Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol 8:250–255
Chung B, Wong V (2004) Pediatric stroke among Hong Kong Chinese subjects. Pediatrics 114:e206–e212
Kiely D, Wolf P, Cupples A, Beiser AS, Myers RH (1993) Familial aggregation of stroke-the Framinghan study. Stroke 24:1366–1371
Brass LM, Isaacsohn J, Merikangas KR, Robinette CD (1992) A study of twins and stroke. Stroke 23:221–223
deVeber G (2003) Arterial ischemic strokes in infants and children: an overview of current approaches. Semin Thromb Hemost 29:567–573
Brattstrom L, Wilcken DE, Ohrvik J et al (1998) Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 98:2520–2526
Frosst P, Blom HJ, Milos R et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113
Zak I, Sarecka-Hujar B, Kopyta I, Emich-Widera E, Marszal E, Wendorff J, Jachowicz-Jeszka J (2009) The T allele of the 677C>T polymorphism of methylenetetrahydrofolate reductase gene is associated with an increased risk of ischemic stroke in Polish children. J Child Neurol 24:1262–1267
Biswas A, Tiwari AK, Ranjan R, Meena A, Akhter MS, Yadav BK, Behari M, Saxena R (2009) Prothrombotic polymorphisms, mutations, and their association with pediatric non-cardioembolic stroke in Asian–Indian patients. Ann Hematol 88:473–478
Kenet G, Sadetzki S, Murad H, Martinowitz U, Rosenberg N, Gitel S, Rechavi G, Inbal A (2000) Factor V Leiden and antiphospholipid antibodies are significant risk factors for ischemic stroke in children. Stroke 31:1283–1288
Rook JL, Nugent DJ, Young G (2005) Pediatric stroke and methylenetetrahydrofolate reductase polymorphisms: an examination of C677T and A1298C mutations. J Pediatr Hematol Oncol 27:590–593
Djordjevic V, Stankovic M, Brankovic-Sreckovic V, Rakicevic L, Radojkovic D (2009) Genetic risk factors for arterial ischemic stroke in children: a possible MTHFR and eNOS gene–gene interplay? J Child Neurol 24:823–827
Morita DC, Donaldson A, Butterfield RJ, Benedict SL, Bale JF Jr (2009) Methylenetetrahydrofolate reductase gene polymorphism and childhood stroke. Pediatr Neurol 41:247–249
Sirachainan N, Sasanakul W, Visudtibhan A, Tapanapruksakul P, Charoenkwan P, Kadegasem P, Udomsubpayakul U, Chuansumrit A (2008) The effect of polymorphisms of MTHFR C677T, A1298C, MS A2756G and CBS 844ins68 bp on plasma total homocysteine level and the risk of ischemic stroke in Thai children. Thromb Res 122:33–37
Akar N, Akar E, Deda G, Sipahi T, Orsal A (1999) Factor V1691 G-A, prothrombin 20210 G-A, and methylenetetrahydrofolate reductase 677 C-T variants in Turkish children with cerebral infarct. J Child Neurol 14:749–751
Herak DC, Antolic MR, Krleza JL, Pavic M, Dodig S, Duranovic V, Brkic AB, Zadro R (2009) Inherited prothrombotic risk factors in children with stroke, transient ischemic attack, or migraine. Pediatrics 123:e653–e660
Komitopoulou A, Platokouki H, Kapsimali Z, Pergantou H, Adamtziki E, Aronis S (2006) Mutations and polymorphisms in genes affecting hemostasis proteins and homocysteine metabolism in children with arterial ischemic stroke. Cerebrovasc Dis 22:13–20
Cardo E, Monrós E, Colomé C, Artuch R, Campistol J, Pineda M, Vilaseca MA (2000) Children with stroke: polymorphism of the MTHFR gene, mild hyperhomocysteinemia, and vitamin status. J Child Neurol 15:295–298
Nowak-Göttl U, Sträter R, Heinecke A, Junker R, Koch HG, Schuierer G, von Eckardstein A (1999) Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood 94:3678–3682
Barreirinho S, Ferro A, Santos M, Costa E, Pinto-Basto J, Sousa A, Sequeiros J, Maciel P, Barbot C, Barbot J (2003) Inherited and acquired risk factors and their combined effects in pediatric stroke. Pediatr Neurol 28:134–138
Prengler M, Sturt N, Krywawych S, Surtees R, Liesner R, Kirkham F (2001) Homozygous thermolabile variant of the methylenetetrahydrofolate reductase gene: a potential risk factor for hyperhomocysteinaemia, CVD, and stroke in childhood. Dev Med Child Neurol 43:220–225
McColl MD, Chalmers EA, Thomas A, Sproul A, Healey C, Rafferty I, McWilliam R, Eunson P (1999) Factor V Leiden, prothrombin 20210G→A and the MTHFR C677T mutations in childhood stroke. Thromb Haemost 81:690–694
Zak I, Sarecka B, Balcerzyk A, Niemiec P, Emich-Widera E, Kopyta I, Marszal E (2004) Methylenetetrahydrofolate reductase gene HinfI 677C→T polymorphism and brain ischemic stroke in children: association pilot-study. Part two. Neurol Dziec 13:31–36
Akar N, Akar E, Ozel D, Deda G, Sipahi T (2001) Common mutations at the homocysteine metabolism pathway and pediatric stroke. Thromb Res 102:115–120
Sirachainan N, Tapanapruksakul P, Visudtibhan A, Chuansumrit A, Cheeramakara C, Atamasirikul K, Chotsuppakarn S, Areekul S (2006) Homocysteine, MTHFR C677 T, vitamin B12, and folate levels in Thai children with ischemic stroke: a case–control study. J Pediatr Hematol Oncol 28:803–808
Cumming AM, Olujohungbe A, Keeney S, Singh H, Hay CR, Serjeant GR (1999) The methylenetetrahydrofolate reductase gene C677T polymorphism in patients with homozygous sickle cell disease and stroke. Br J Haematol 107:569–571
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc 283:2008–2012
Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM (2006) Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol 6:50
Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM (2008) MIX: comprehensive free software for meta-analysis of causal research data. Version 1.7. http://mix-for-meta-analysis.info
Kang SS, Wong PW, Bock HG (1991) Intermediate hyperhomocysteinemia resulting from compound heterozygosity of methylenetetrahydrofolate reductase mutations. Am J Hum Genet 48:546–551
Fowler B (1997) Disorders of homocysteine metabolism. J Inherit Metab Dis 20:270–285
Signorello MG, Segantin A, Passalacqua M, Leoncini G (2009) Homocysteine decreases platelet NO level via protein kinase C activation. Nitric Oxide 20:104–113
de Bree A, Verschuren WM, Bjørke-Monsen AL, van der Put NM, Heil SG, Trijbels FJ, Blom HJ (2003) Effect of the methylenetetrahydrofolate reductase 677C→T mutation on the relations among folate intake and plasma folate and homocysteine concentrations in a general population sample. Am J Clin Nutr 77:687–693
Alsayouf H, Zamel KM, Heyer GL, Khuhro AL, Kahwash SB, de los Reyes EC (2011) Role of methylenetetrahydrofolate reductase gene (MTHFR) 677C>T polymorphism in pediatric cerebrovascular disorders. J Child Neurol 26:318–321
Unal E, Mutlu FT, Karakukcu M (2012) The importance of MTHFR polymorphisms in pediatric cerebral stroke. Childs Nerv Syst 28:13
Kim NK, Kang GD, Kim HJ (2002) Genetic polymorphisms of 5,10-methylenetetrahydrofolate reductase (MTHFR C677T and A1298C) in healthy Koreans. Korean J Genet 24:227–234
Franco RF, Araujo AG, Guerriero JF, Elion J, Zago MA (1998) Analysis of the 677 CT mutation of the methylenetetrahydrofolate reductase gene in different ethnic groups. Thromb Haemost 79:119–121
Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG (2002) MTHFR Studies Collaboration Group. MTHFR 677C→T polymorphism and risk of coronary heart disease: a meta-analysis. J Am Med Assoc 288:2023–2031
McColgan P, Sharma P (2008) The genetics of carotid dissection: meta-analysis of a MTHFR/C677T common molecular variant. Cerebrovasc Dis 25:561–565
Banerjee I, Gupta V, Ganesh S (2007) Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet 52:205–219
Cronin S, Furie KL, Kelly PJ (2005) Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke 36:1581–1587
Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A, Furie KL (2002) Homocysteine, MTHFR 677C→T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology 59:529–536
Wu AH, Tsongalis GJ (2001) Correlation of polymorphisms to coagulation and biochemical risk factors for cardiovascular diseases. Am J Cardiol 87:1361–1366
Sarecka-Hujar B, Zak I, Krauze J (2008) Carrier-state of two or three polymorphic variants of MTHFR, IL-6 and ICAM1 genes increases the risk of coronary artery disease. Kardiol Pol 66:1269–1277
Den Heijer M, Lewington S, Clarke R (2005) Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost 3:292–299
Lynch JK, Hirtz DG, DeVeber G, Nelson KB (2002) Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 109:116–123
Acknowledgments
Preliminary data was presented on the 19th European Stroke Conference held in Barcelona, Spain in May 25–28th 2010. The following researchers kindly provided additional information from their studies: Nongnuch Sirachainan, Nejat Akar, Ulrike Nowak-Göttl, Guy Young.
Conflict of interest
Authors declare that there is no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sarecka-Hujar, B., Kopyta, I., Pienczk-Reclawowicz, K. et al. The TT genotype of methylenetetrahydrofolate reductase 677C>T polymorphism increases the susceptibility to pediatric ischemic stroke: meta-analysis of the 822 cases and 1,552 controls. Mol Biol Rep 39, 7957–7963 (2012). https://doi.org/10.1007/s11033-012-1641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1641-9