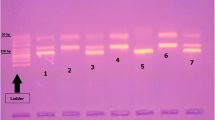
Abstract
Catalase is the main regulator of hydrogen peroxide metabolism. In vitiligo patients there are conflicting data on its activity and no data on the effect of −262C>T polymorphism in the catalase gene. Blood catalase activity, −262C>T polymorphism and acatalasemia mutations were examined in 75 vitiligo patients and in 162 controls, in Hungary. We measured blood catalase activity and conducted analyses with PCR-SSCP, polyacrylamide gel electrophoresis and silver staining in combination with RFLP and nucleotide sequencing. Comparison of the wild (CC) genotype and the mutant (TT) genotype in the vitiligo patients revealed a non significant (P > 0.19) increase in blood catalase. Male controls with the CT genotype had significantly (P < 0.04) lower blood catalase activity than CC genotype controls. Female vitiligo patients with CC genotype had lower (P < 0.04) blood catalase than female controls. The frequency of wild genotype (CC) and C alleles is significantly (P < 0.04) decreased in Hungarian controls when compared to controls in Slovenia, Morocco, UK, Greece, Turkey, USA, China. The detection of a novel acatalasemia mutation (37C>T in exon 9) and the 113G>A (exon 9) mutation in Hungary are further proofs of genetic heterogeneity origin of acatalasemia mutations. In conclusion, the −262 C>T polymorphism has a reverse effect on blood catalase in vitiligo patients and in controls. In controls the mutant genotypes and alleles are more frequent in Hungary than in several other populations. The new acatalasemia mutations are further examples of heterogeneity of acatalasemia.




Similar content being viewed by others
References
Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, Hibberts NA (1999) In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Invest Dermatol Symp Proc 4(1):91–96
Schallreuter KU, Gibbons NCJ, Zothner C, Abou Elloof MM, Wood JM (2007) Hydrogen peroxide-mediated oxidative stress disrupts calcium bindingon calmodulin: more evidence for oxidative stress in vitiligo. Biochem Biophys Res Com 360:70–75
Schallreuter KU, Rubsam K, Gibbons NCJ, Maitland DJ, Chavan B, Zothner C, Rokos H, Wood JM (2008) Methionine sulfoxide reductases a and b are deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J Invest Dermatol 128(4):808–815
Schallreuter KU, Elwary S (2007) Hydrogen peroxide regulates the cholinerg signal in a concentration dependent manner. Biochem Biophys Res Com 360(1):70–75
Wood JM, Schallreuter KU (2006) UVA-irradiated pheomelanin alters the structure of catalase and decreases its activity in human skin. J Invest Dermatol 126:13–14
Maresca V, Flori E, Briganti S, Camera E, Cario-Andre’ M, Taieb A, Picardo M (2006) UVA-induced modification of catalase charge properties in the epidermis is correlated with the skin phototype. J Invest Dermatol 126:182–190
Mueller S, Riedel HD, Stremmel W (1997) Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 90:4973–4978
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator or ageing signal? Nat Rev Mol Cell Biol. 8:722–728
Góth L, Eaton WJ (2000) Hereditary catalase deficiencies and increased risk of diabetes. Lancet 356:1820–1821
Góth L, Vitai M, Rass P, Sükei E, Páy A (2005) Detection of a novel familial catalase mutation (Hungarian type D) and the possible risk of inherited catalase deficiency for diabetes mellitus. Electrophoresis 26:1646–1649
Casp CB, She JX, McCormack WT (2002) Genetic association of catalase gene (CAT) with vitiligo susceptibility. Pigment Cell Res 15:62–66
Gavalas NG, Akhtar S, Gawkrodger DJ, Watson PF, Weetman AP, Kemp EH (2006) Analysis of allelic variants in the catalase gene in patients with the skin depigmentation disorder vitiligo. Biochem Biophys Res Com 345:1586–1591
Park HH, Ha E, Uhm YK, Jin SY, Chung JH, Lee MH (2006) Association study between catalase gene polymorphisms and the susceptibility to vitiligo in Korean population. Exper Dermatol 15:377–380
Shajil EM, Laddha NC, Chatterjee S, Gani AR, Malek RA, Shah BJ, Begum R (2006) Association of catalase T/C exon 9 and glutathione peroxidase codon 200 polymorphisms in relation to their activities and oxidative stress with vitiligo susceptibility in Gujarat population. Pigment Cell Res 20:405–407
Góth L (2000) Lipid and carbohydrate metabolism in acatalasemia. Clin Chem 46:564–566
Góth L, Vitai M (2003) The effects of hydrogen peroxide promoted by homocysteine and inherited catalase deficiency on human hypocatalasemic patients. Free Rad Biol Med 35:882–888
Parboosingh JS, Rousseau M, Rogan F, Amit ZH, Chertkow H, Johnson WG, Manganaro F, Schipper HN, Currant TJ, Stoessl J, Rouleau G (1995) Absence of mutations in superoxide dismutase and catalase genes in patients with Parkinson’s disease. Arch Neurol 52:1160–1163
Konings A, Van Laer L, Pawelczyk M, Carlsson PI, Bondeson ML, Rajkowska E, Dudarewicz A, Vandeveldel A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, Van Camp GS (2007) Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet 16:1872–1883
Oh B, Kim SY, Kim DJ, Lee YJ, Lee JK, Kimm K, Park BL, Shin HD, Kim TH, Park EK, Koh JM, Kim GS (2007) Association of catalase gene polymorphism with bone mineral density and bone turnover markers in postmenopausal women. J Med Genet 44(1):e62
Góth L, Bigler NW (2007) Catalase deficiency may complicate urate oxidase (rasburicase) therapy. Free Rad Res 41(9):953–955
Abu-Alfa AK, Younes A (2010) Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis 55(5):S1–S13
Hamada Y, Kameyama Y, Iizuka T, Ishizaki T, Nishiyama T, Isshiki A (2004) Methemoglobinemia from hydrogen peroxide in a patient with acatalasemia. Anesthesiology 101(1):247–248
Góth L, Rass P, Pay A (2004) Catalase enzyme mutations and their association with diseases. Mol Diagn 8(3):141–149
Wally V, Buchroithner B, Klausegger A, Laimer M, Hintner H, Bauer JW (2004) Acatalasemia: identification and characterization of a novel missense mutation. J Invest Dermatol 122:A85–85, 506 (meeting abstract)
Ogata M (2001) Acatalasemia. Hum Genet 86:331–340
Aebi H, Heiniger JP, Bütler R, Hassig A (1961) Two cases of acatalasemia in Switzerland. Experientia 17:466
Góth L (1992) Two cases of acatalasemia in Hungary. Clin Chim Acta 207:155–158
Takahara S, Miyamoto H (1948) Three cases of progressive oral gangrene due to lack of catalase in the blood (in Japanese). J Otol Soc Jpn 51:163–164
Schallreuter KU, Wood JM, Berger J (1991) Low catalase levels in the epidermis of patients with vitiligo. J Invest Dermatol 97:1081–1085
Forsberg L, Lyrenas L, de Faire U, Morgenstern R (2001) A common functional C–T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase level. Free Radical Biol Med 30(5):500–505
Nosikov VV (2004) Genomics of type 1 diabetes mellitus and its late complications. Mol Biol 38(1):128–139
Zotova EV, Savostyanov KV, Chistyakov DA, Bursa TP, Galeev IV, Strokov IA, Nosikov VV (2004) Association polymorphic markers of the antioxidant enzyme genes with diabetic polyneuropathy in type 1 diabetes mellitus. Mol Biol 38(2):200–204
Hovnik T, Dolzan V, Bratina NU, Podkrajsek KT, Battelino T (2009) Genetic polymorphisms in a gene encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care 32(12):2258–2262
Chistatiakov DA, Savostanov KV, Turakulov RI (2004) A new type 1 diabetes susceptibility locus containing the catalase gene (chromosome 11p13) in a Russian population. Diabetes Metab Res Rews 20:219–224
Pasak R, Cooper J, Walker NM, Nutland S, Hutching J, Dunger DB, Nejentsev S, Todd JA (2006) No evidence for a major effect of two common polymorphisms of the catalase gene in type 1 diabetes susceptibility. Diab Metab Res Rev 22:356–360
Szelestei T, Bahrung S, Wagner Z, Aydin A, Molnar GA, Kocsis B, Nagy J, Whittman I (2005) Serum levels of arginine analogues and glutathione peroxidase and catalase gene variants in type 2 diabetes mellitus patients. Diabet Med 22:356–357
Goulas A, Fidani L, Kotsis A, Mirtsou V, Petersen RC, Tangalos E, Hardy J (2002) An association study of a functional catalase gene polymorphism, −262C>T, and patients with Alzheimer’s diseases. Neurosci Lett 330:210–212
Ezzikouri S, Feydi AEE, Afifi R, Benazzouz M, Hassar M, Pineau P, Benjelloun S (2010) Polymorphisms in antioxidant defence genes and susceptibility to hepatocellular carcinoma in a Moroccon population. Free Rad Res 44(2):208–210
Kosmidou M, Hatzitolios A, Molyva D, Raikos N, Savopoulos C, Daferera N, Kokkas V, Goulas A (2009) An association study between catalase −262C>T gene polymorphism, sodium-lithium countertransport activity, insulin resistance, blood lipid parameters and their response to atorvastatin, in Greek dyslipidaemic patients and normolipidaemic controls. Free Rad Res 43(4):385–389
Wen JK, Osumi T, Hashimoto T (1990) Molecular analysis of human acatalasemia. J Mol Biol 211:383–393
Franko A, Dolizan V, Arneric N, Dodic-Fikfark M (2008) Asbestosis and catalase gene polymorphism. Arhiv zo Higijene Rada I Toksikologiju 59(4):233–240
Suzen SH, Gucyner E, Sakalli O, Uckum Z, Kose G, Ustel D, Duydu Y (2010) CAT-262T and GPXI Pro198Leu polymorphism in a Turkish population. Mol Biol Rep 37:87–92
Ahn J, Nowel S, McCann SE, Yu J, Carter L, Lang NP, Kadlubar FF, Ratnasinghe LD, Ambrosone CB (2006) Association between catalase phenotype and genotype: modification by epidemiologic factors. Cancer Epidemol Biomarkers Rev 15(6):1217–1222
Liang B, Zhang A-H, Xi X-G, Zhang B-X, Huang X-X (2009) Relationship between myeloperoxidase and catalase genetic polymorphism and their activities with arsenic poisoning caused by coal-burning. Chin J Endemiol 28(3):272–275
NCI SNP500. http://snp500cancer.nci.nih.gov
Putnam CD, Arvai AS, Bourne Y, Tainer JA (2000) Active and inhibited human catalase structures: ligand and NADPH-binding and catalytic mechanism. J Mol Biol 296:295–309
Diaz A, Horjales E, Rudiña-Piñera E, Arreola R, Hansberg W (2004) Unusual Cys–Tyr covalent bond in a large catalase. J Mol Biol 342:971–985
Góth L, Csordás M, Kósa Zs, Simics E (2011) A weak association of blood catalase activity and +22348C → T polymorphism of the catalase gene in Hungarian female vitiligo patients. Clin Exper Med(Budapest) 5:1–7
Acknowledgments
This study was supported by grants from OTKA [Hungarian Research Foundation K71902] and Zsigmond Diabetes Foundation [Hungarian Academy of Science, Department of Medicine]. The authors thank WN. Bigler (San Francisco State University, San Francisco, USA) and É. Gombos (Department of Clinical Biochemistry and Molecular Pathology, University of Debrecen, Debrecen, Hungary), K. Kocsordi (Department of Biomedical Laboratory and Imaging Science, University of Debrecen, Debrecen, Hungary) for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zsuzsanna Kósa, Zsolt Fejes, Teréz Nagy, Melinda Csordás, Enikő Simics, Éva Remenyik, László Góth contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kósa, Z., Fejes, Z., Nagy, T. et al. Catalase −262C>T polymorphisms in Hungarian vitiligo patients and in controls: further acatalasemia mutations in Hungary. Mol Biol Rep 39, 4787–4795 (2012). https://doi.org/10.1007/s11033-011-1272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1272-6