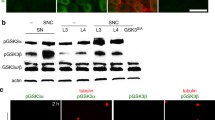
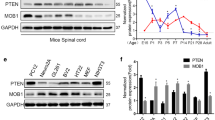
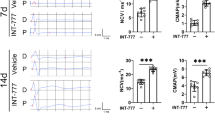
Abstract
Spinal cord injury (SCI) is a serious neurotrauma that can lead to life-long disability; to date, no suitable therapeutic strategy exists. Axons do not regenerate after SCI in adult mammals and loss of skeletal muscle mass occurs very rapidly after SCI. Promotion of neurite growth through improving the extracellular environment allows only a limited degree of axon regeneration. The phosphatidylinositol-3 kinase (PI3K)/Akt pathway and its downstream targets (“mammalian target of rapamycin,” mTOR, and glycogen synthase kinase-3), which regulate cell growth and proliferation in many tissues, have been suggested to play an important role in regulation of the intrinsic axonal regeneration and muscle hypertrophy. This review is focused on recent progress in our understanding of the PI3K pathway in the modulation of axonal regeneration and muscle hypertrophy after SCI.

Similar content being viewed by others
References
Dudley GA, Castro MJ, Rogers S, Apple DF Jr (1999) A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80:394–396
Leger B, Vergani L, Soraru G, Hespel P, Derave W, Gobelet C et al (2006) Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1. FASEB J 20:583–585
Lynch GS (2001) Therapies for improving muscle function in neuromuscular disorders. Exerc Sport Sci Rev 29:141–148
Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D et al (2005) A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43:408–416
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319–370
Goldberg JL, Klassen MP, Hua Y, Barres BA (2002) Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296:1860–1864
Filbin MT (2006) Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci 361:1565–1574
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99:467–472
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Jaworski J, Sheng M (2006) The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 34:205–219
Goswami R, Kilkus J, Dawson SA, Dawson G (1999) Overexpression of Akt (protein kinase B) confers protection against apoptosis and prevents formation of ceramide in response to pro-apoptotic stimuli. J Neurosci Res 57:884–893
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218
Welsh GI, Stokes CM, Wang X, Sakaue H, Ogawa W, Kasuga M et al (1997) Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett 410:418–422
Sun F, He Z (2010) Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol 20:510–518
Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S et al (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110:177–189
Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H et al (2003) GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11:895–904
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H et al (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD et al (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29:541–551
Woodgett JR (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9:2431–2438
Alabed YZ, Pool M, Ong Tone S, Sutherland C, Fournier AE (2010) GSK3 beta regulates myelin-dependent axon outgrowth inhibition through CRMP4. J Neurosci 30:5635–5643
Chen W, Chen M, Barak LS (2010) Development of small molecules targeting the Wnt pathway for the treatment of colon cancer: a high-throughput screening approach. Am J Physiol Gastrointest Liver Physiol 299:G293–G300
Rhoads RE (1999) Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem 274:30337–30340
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN et al (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
Consortium TECTS (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315
Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW (1994) Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci USA 91:11477–11481
Jeno P, Ballou LM, Novak-Hofer I, Thomas G (1988) Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci USA 85:406–410
Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171
Jefferies HB, Reinhard C, Kozma SC, Thomas G (1994) Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA 91:4441–4445
Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15:807–826
Asante CO, Wallace VC, Dickenson AH (2010) Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J Pain 11:1356–1367
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963–966
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I et al (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13:1075–1081
Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW (2010) PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci 30:9306–9315
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H et al (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166:213–223
Hu LY, Sun ZG, Wen YM, Cheng GZ, Wang SL, Zhao HB et al (2010) ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience 169:1046–1062
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Im E, von Lintig FC, Chen J, Zhuang S, Qui W, Chowdhury S et al (2002) Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene 21:6356–6365
Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M et al (2009) The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci 29:1093–1104
Dann SG, Thomas G (2006) The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 580:2821–2829
Reiling JH, Sabatini DM (2008) Increased mTORC1 signaling UPRegulates stress. Mol Cell 29:533–535
Jiang H, Guo W, Liang X, Rao Y (2005) Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 120:123–135
Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K (2005) GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120:137–149
Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ et al (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7:793–803
Dill J, Wang H, Zhou F, Li S (2008) Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 28:8914–8928
Cyranoski D (2007) Chinese network to start trials of spinal surgery. Nature 446:476–477
Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T et al (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52:981–996
Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM et al (2002) WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron 35:1043–1056
Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42:897–912
Liu M, Bose P, Walter GA, Thompson FJ, Vandenborne K (2008) A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord 46:488–493
Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E (1986) High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil 67:445–450
Bauman WA, Spungen AM (2000) Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 11:109–140
Kraus JF, Greenland S (1983) Survival from spinal cord injury. J Chronic Dis 36:297–298
Leger B, Senese R, Al-Khodairy AW, Deriaz O, Gobelet C, Giacobino JP et al (2009) Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40:69–78
Dreyer HC, Glynn EL, Lujan HL, Fry CS, DiCarlo SE, Rasmussen BB (2008) Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway. J Appl Physiol 104:27–33
Ladner KJ, Caligiuri MA, Guttridge DC (2003) Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278:2294–2303
Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL et al (2005) TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19:362–370
Glass DJ (2010) PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol 346:267–278
Kimball SR, Farrell PA, Jefferson LS (2002) Invited Review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93:1168–1180
Rochat A, Fernandez A, Vandromme M, Moles JP, Bouschet T, Carnac G et al (2004) Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol Biol Cell 15:4544–4555
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y et al (2005) Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280:2737–2744
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO et al (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15:1537–1545
Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X (2008) Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J 55:11–21
Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7242
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270
Hou S, Xu Q, Tian W, Cui F, Cai Q, Ma J et al (2005) The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods 148:60–70
Lohmeyer JA, Essmann E, Richerson SJ, Hagel C, Egana JT, Condurache A et al (2008) Use of erythropoietin as adjuvant therapy in nerve reconstruction. Langenbecks Arch Surg 393:317–323
Mao X, Zeng X, Wang J, Qiao S (2010) Leucine promotes leptin receptor expression in mouse C2C12 myotubes through the mTOR pathway. Mol Biol Rep 38(5):3201–3206. doi:10.1007/s11033-010-9992-6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, T., Qi, Y., Li, Y. et al. PI3 Kinase regulation of neural regeneration and muscle hypertrophy after spinal cord injury. Mol Biol Rep 39, 3541–3547 (2012). https://doi.org/10.1007/s11033-011-1127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1127-1