Abstract
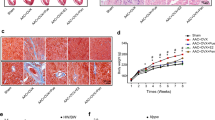
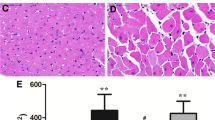
Our findings indicate that in ovariectomized female rats abdominal aortic constriction led to significant increases in left ventricular mass, myocyte diameter and heart weight/body weight (HW/BW) value, and decreases in interventricular septal thickness at diastole (IVSd), left ventricular percent fractional shortening (FS) and ejection fraction (EF). These pathophysiological alterations were largely reversed by administration with 17β-estradiol for eight weeks. Furthermore, the enhanced expression of extracellular signal-regulated kinases 1/2 and decreased expression of caveolin-3 were found in left ventricle of AAC group. 17β-estradiol (E2) administration increased the expression of caveolin-3 and reduced the level of ERK phosphorylation in these pressure-overloaded rats. Moreover, in cultured neonatal rat cardiomyocytes, E2 inhibited the hypertrophic response to angiotensin II. This effect was reinforced by the addition of extracellular signal-regulated kinases 1/2 inhibitor PD98059, but was impaired when the cells were pretreated with caveolae disruptor, methyl-β-cyclodextrin (M-β-CD). In conclusion, our data indicate that estrogen attenuates the hypertrophic response induced by pressure overload through down-regulation of extracellular signal-regulated kinases 1/2 phosphorylation and up-regulation of caveolin-3 expression.



Similar content being viewed by others
Abbreviations
- HW/BW:
-
Heart weight/body weight
- E2 :
-
17β-estradiol
- ERK1/2:
-
Extracellular signal-regulated kinases 1/2
- M-β-CD:
-
Methyl-β-cyclodextrin
- Ang II:
-
Angiotensin II
- LVH:
-
Left ventricular hypertrophy
- ERs:
-
Estrogen receptors
- MAPK:
-
Mitogen-activated protein kinase
- PMSF:
-
Phenylmethylsulfonyl fluoride
- OVX:
-
Ovariectomized
- AAC:
-
Abdominal aorta constriction operation
- LVDd:
-
Left ventricular end-diastolic diameters
- LVDs:
-
Left ventricular end-systolic diameters
- IVSd:
-
Interventricular septal thickness at diastole
- LVPWD:
-
Left ventricular posterior wall diameter
- FS:
-
Fractional shortening
- EF:
-
Ejection fraction
References
Zou XJ, Yang L, Yao SL (2008) Propofol depresses angiotensin II-induced cardiomyocyte hypertrophy in vitro. Exp Biol Med (Maywood) 233:200–208
Kannel WB (2000) Incidence and epidemiology of heart failure. Heart Fail Rev 5:167–173
Lim WK, Wren B, Jepson N, Roy S, Caplan G (1999) Effect of hormone replacement therapy on left ventricular hypertrophy. Am J Cardiol 83:1132–1134, A1139
Maris ME, Melchert RB, Joseph J, Kennedy RH (2005) Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol 32:35–39
Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B et al (2006) Estrogen receptor beta protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol 26:1524–1530
Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J et al (1997) Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 272:29337–29346
Thomas CM, Smart EJ (2008) Caveolae structure and function. J Cell Mol Med 12:796–809
Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M et al (1996) Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271:15160–15165
Meldrum DR, Wang M, Tsai BM, Kher A, Pitcher JM, Brown JW et al (2005) Intracellular signaling mechanisms of sex hormones in acute myocardial inflammation and injury. Front Biosci 10:1835–1867
Bueno OF, De Windt LJ, Lim HW, Tymitz KM, Witt SA, Kimball TR et al (2001) The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res 88:88–96
Kim N, Kim H, Youm JB, Park WS, Warda M, Ko JH et al (2006) Site specific differential activation of ras/raf/ERK signaling in rabbit isoproterenol-induced left ventricular hypertrophy. Biochim Biophys Acta 1763:1067–1075
Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J et al (2001) Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103:670–677
Fujita T, Toya Y, Iwatsubo K, Onda T, Kimura K, Umemura S et al (2001) Accumulation of molecules involved in alpha1-adrenergic signal within caveolae: caveolin expression and the development of cardiac hypertrophy. Cardiovasc Res 51:709–716
Piech A, Massart PE, Dessy C, Feron O, Havaux X, Morel N et al (2002) Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 282:H219–H231
Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J et al (2002) Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem 277:38988–38997
Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T et al (1998) Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett 428:205–211
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL et al (2003) Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17:1263–1293
Jones NC, Fedorov YV, Rosenthal RS, Olwin BB (2001) ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J Cell Physiol 186:104–115
Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y (2004) Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286:H1845–H1852
Kawamura S, Miyamoto S, Brown JH (2003) Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278:31111–31117
Bellott AC, Patel KC, Burkholder TJ (2005) Reduction of caveolin-3 expression does not inhibit stretch-induced phosphorylation of ERK2 in skeletal muscle myotubes. J Appl Physiol 98:1554–1561
Doll D, Sarikas A, Krajcik R, Zolk O (2007) Proteomic expression analysis of cardiomyocytes subjected to proteasome inhibition. Biochem Biophys Res Commun 353:436–442
Patten RD, Aronovitz MJ, Einstein M, Lambert M, Pandian NG, Mendelsohn ME et al (2003) Effects of angiotensin II receptor blockade versus angiotensin-converting-enzyme inhibition on ventricular remodelling following myocardial infarction in the mouse. Clin Sci (Lond) 104:109–118
Sharkey LC, Holycross BJ, Park S, Shiry LJ, Hoepf TM, McCune SA et al (1999) Effect of ovariectomy and estrogen replacement on cardiovascular disease in heart failure-prone SHHF/Mcc- fa cp rats. J Mol Cell Cardiol 31:1527–1537
van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA (2001) 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104:1419–1423
Garrington TP, Johnson GL (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11:211–218
Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R et al (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19:6341–6350
Hunter JJ, Tanaka N, Rockman HA, Ross J Jr, Chien KR (1995) Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem 270:23173–23178
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7:589–600
Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y et al (2008) Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol 44:123–130
Kikuchi T, Oka N, Koga A, Miyazaki H, Ohmura H, Imaizumi T (2005) Behavior of caveolae and caveolin-3 during the development of myocyte hypertrophy. J Cardiovasc Pharmacol 45:204–210
Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M et al (2001) Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 276:21425–21433
Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M et al (2000) Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA 97:9689–9694
Volonte D, Peoples AJ, Galbiati F (2003) Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol Biol Cell 14:4075–4088
Galbiati F, Volonte D, Engelman JA, Scherer PE, Lisanti MP (1999) Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J Biol Chem 274:30315–30321
Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE et al (1999) Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 19:7289–7304
Liu HM, Zhao XF, Guo LN, Tan Z, Wang TH (2007) Effects of caveolin-1 on the 17beta-estradiol-mediated inhibition of VSMC proliferation induced by vascular injury. Life Sci 80:800–812
Tan Z, Lin GP, Wang TH (2004) Possible involvement of caveolin-1 in the inhibition of endothelin-1 induced proliferation of vascular smooth muscle cells by 17beta-estradiol. Sheng Li Xue Bao 56:379–383
Sheng H, Zhu J, Wu X, Yang D, Zhang J (2007) Angiotensin-converting enzyme inhibitor suppresses activation of calcineurin in renovascular hypertensive rats. Hypertens Res 30:1247–1254
Tutor AS, Penela P, Mayor F Jr (2007) Anti-beta1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res 76:51–60
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, YH., Tan, Z., Fu, XD. et al. 17beta-estradiol attenuates pressure overload-induced myocardial hypertrophy through regulating caveolin-3 protein in ovariectomized female rats. Mol Biol Rep 38, 4885–4892 (2011). https://doi.org/10.1007/s11033-010-0630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0630-0