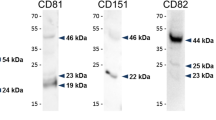
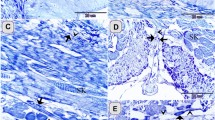
Abstract
The tetra-membrane-spanning protein, CD9 is a 24–27 kDa cell surface glycoprotein expressed in a wide variety of human cells being involved in a variety of cell processes, including signaling, adhesion, motility, fertilization and tumor cells metastasis. By means of a polyclonal antibody (N1) raised against recombinant swine CD9 protein, we studied the immunohistochemical expression of CD9 on different normal swine tissues. Immunochemistry shows that swine CD9 was distribute in a similar form than in human tissues, being present on epithelial cells of lung, liver, kidney, skin, tonsil, testis (epididymo), gut mucosa, uterus and mama. Furthermore, polyclonal antibody against swine CD9 reacts with white matter from cerebrum and cerebellum, peripheral nerves fibers and Hassal corpuscle from thymus and ovum. Platelets react strongly with our antibody, but monocytes and neutrophils react lightly. These results suggest that CD9 antigen should play a similar functional role in swine and human and therefore studies on CD9 on swine as an animal model would allow new knowledge about its role in adhesion, fertilization and tumor metastasis among other important biomedical processes.



Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DIG:
-
Digoxigenin
- DMSO:
-
Dimethylsulphoxide
- EGFR:
-
Epidermal growth factor receptor
- MT1-MMP:
-
Membrane type-1 matrix metalloproteinase
- PBL:
-
Peripheral blood lymphocyte
- PBMC:
-
Peripheral blood mononuclear cell
- PBS:
-
Phosphate buffered saline
- PRP:
-
Porcine platelet-rich plasma
- SDS:
-
Sodium dodecylsulfate
- TGF:
-
Transforming growth factor
References
Lanza F, Wolf D, Fox CF et al (1991) cDNA cloning and expression of platelet p25/CD29. J Biol Chem 266:10638–10645
Hemler ME (2003) Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Develop Biol 19:397–422
Maecker HT, Todd SC, Levy S (1997) Tetraspanin superfamily: molecular facilitators. FASEB J 11:428–442
Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114:4143–4151
Boucheix C, Rubinstein E (2001) Tetraspanins. Cell Mol Life Sci 58:1189–1205
Hemler ME (2001) Specific tetraspanin functions. J Cell Biol 155:1103–1107
Kersey JH, Lebein T, Abramson CS et al (1981) p24 a human leukemia associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody Ba2. J Exp Med 153:726–731
Boucheix C, Benoit P (1988) CD9 antigen: will platelet physiology help to explain the function of a surface molecule during hemopoietic differentiation. Nouv Rev Fr Hematol 30:201–202
Nakamura Y, Iwamoto R, Mekada E (1996) Expression and distribution of CD9 in myelin of the central and peripheral nervous systems. Am J Pathol 149:575–583
Ash RC, Jansen J, Kersey JH, LeBien TW, Zanjani ED (1982) Normal human pluripotential and committed hematopoietic progenitors do not express the p24 antigen detected by monoclonal antibody BA-2: implications for immunotherapy of lymphocytic leukemia. Blood 60:1310
Heinz M, Huang CA, Emery DW, Giovino MA, LeGuern A, Kurilla-Mahon B, Theodore P, Arn JS, Sykes M, Mulligan R, Down JD, Sachs DH, Goodell MA (2002) Use of CD9 expression to enrich for porcine hematopoietic progenitors. Exp Hematol 30(7):809–815
García-López MA, Barreiro O, García-Díez A, Sánchez-Madrid F, Penas PF (2005) Role of tetraspanins CD29 and CD151 in primary melanocyte motility. J Invest Dermatol 125:1001–1009
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6:801–811
Longhurts CM, Jacobs JD, White MM et al (2002) Chinese hamster ovary cell motility to fibronectin is modulated by the second extracellular loop of CD9: identification of a putative fibronectin binding site. J Biol Chem 277:32445–32552
Jennings LK, Fox CF, Kouns WC et al (1990) The activation of human platelets mediated by anti-human platelet p24/CD9 monoclonal antibodies. J Biol Chem 265(7):3815–3822
Rubinstein F, Le Nanour F, Billar M, Prenant M, Boucheix C (1994) CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol 24:3005–3013
Kotha J, Longhurst C, Appling W, Jennings LK (2008) Tetraspanin CD9 regulates b1 integrin activation and enhances motility to fibronectin via a PI-3 kinase-dependent pathway. Exp Cell Res 314:1811–1822
Cook GA, Longhurst CM, Grgurevich S, Crossno JT, Jennings LK (2002) Identification of CD9 extracellular domains important in regulation of CHO cell adhesion to fibronectin and fibronectin pericellular matrix assembly. Blood 100(13):4502–4511
Lafleur MA, Xu D, Helmer ME (2009) Tetraspanin protein regulates membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell 20:2030–2040
Stipp CS, Kolesnikova TV, Helmer ME (2003) Functional domains in tetraspanin proteins. Trends Biochem Sci 28:106–112
Cajot JF, Sordat I, Sylvester T, Sordat B (1997) Differential display cloning identifies motility-related protein (MRP1/CD9) as highly expressed in primary compared to metastatic human colon cancer cells. Cancer Res 57:2593–2597
Sauer G, Windisch J, Kurzeder C, Heilmann V, Kreinberg R, Deisller H (2003) Progression of cervical carcinoma is associated with down-regulation of CD9 but strong local re-expression at sites of transendothelial invasion. Clin Cancer Res 9:6426–6431
Hori H, Yano S, Koufuji K, Takeda J, Shirouzu K (2004) CD9 expression in gastric cancer and its significance. J Surg Res 117:208–215
Takeda T, Hattori N, Tokuhara T, Nishimura Y, Yokoyama M, Miyake M (2007) Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res 67:1744–1749
Miyake M, Nakano K, Itoi SI, Koh T, Taki T (1996) Motility related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res 56:1244–1249
Sakamoto K, Nakamura Y, Nakashima (2004) Immunohistochemical distribution of CD9 in parotid gland tumors. Auris Nasus Larynx 31(1):49–55
Murayama Y, Shinomura Y, Oritani K et al (2008) The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol 216(1):135–143
Imhoff I, Gasper WJ, Derynk R (2008) Association of tetraspanin CD9 with transmembrane TGFa and cytoskeletal organization. J Cell Sci 121:2265–2274
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A (2000) The gamete fusion process is defective in eggs of CD9-deficient mice. Nat Genet 24:279–282
Chen MS, Tung KSK, Coonrod SA, Takahashi Y, Bigler D, Chang A, Tamashita Y, Kincade PW, Herr JC, White JM (1999) Role of the integrin associated protein CD9 in binding between sperm ADAM 2 and the egg integrin a6b1: implications for murine fertilization. Proc Natl Acad Sci USA 96:11830–11835
Takhashi Y, Bigler D, Ito Y, White JM (2001) Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of the b1 integrin-associated proteins CD9, CD81, and CD98. Mol Biol Cell 12:809–820
Wong GE, Zhu X, Prater CE, Oh E, Evans JP (2001) Analysis of fertilin (ADAM 1)-mediated sperm–egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J Biol Chem 276:24937–24945
Zhu X, Evans JP (2002) Analysis of the roles of RGD-binding integrins, a4/a9 integrins, α6 integrins, and CD9 in the interaction of the fertilin b (ADAM) disintegrin domain with the mouse egg membrane. Biol Reprod 66:1193–1202
Li Y-H, Hou Yi, Ma Wei, Yuan Jin-Xiang, Zhang Dong, Sun Qing-Yuan, Wang Wei-Hua (2004) Localization of CD9 in pig oocytes and its effects on sperm–egg interaction. Reproduction 127:151–157
Willett BJ, Hosie MJ, Jarrett O, Neil JC (1994) Identification of a putative cellular receptor for feline immunodeficiency virus as the feline homologue of CD9. Immunology 81:228–233
Hosie M, Willeft BJ, Dunsford TH, Jarrett O, Neil JC (1993) A monoclonal antibody which blocks infection with feline immunodeficiency virus identifies a possible non-CD4 receptor. J Virol 1667–1671
Yubero N, Jiménez-Marín A, Yerle M, Morera L, Barbancho M, Llanes D, Garrido JJ (2003) Molecular cloning, expression pattern and chromosomal mapping of pig CD9 antigen. Cytogenet Genome Res 101:143–146
Tanaka H, Kobayashi E (2006) Education and research using experimental pigs in a medical school. J Artif Organs 9:136–143
Serebruany VL, Ordonez JV, Yurovsky VV, Gurbel PA (1998) The crossreactivity of human vs swine platelet surface antigens: similarity of glycoproteins Ib and IIIa, but not IIb/IIIa complex. J Thromb Thrombolysis 5:37–41
Sincock PM, Mayrhofer G, Asham LK (1997) Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, Cd63 and α5β1 integrin. J Histochem Cytochem 45:515–525
Nakamura Y, Handa K, Iwamoto R, Tsukamoto T, Takahasi M, Mekada E (2001) Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin α3β1 in normal human tissues. J Histochem Cytochem 49(4):439–444
Jones NH, Borowitz MJ, Metzgar RS (1982) Characterization and distribution of a 24, 000-molecular weight antigen defined by a monoclonal antibody (DU-ALL-1) elicited to common acute lymphoblastic leukaemia (cALL) cells. Leuk Res 4:449–464
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Rossler K, Neuchrist C, Kitz K, Scheiner O, Kraft D, Lassman H (1992) Expression of leukocyte adhesion molecules at the human blood brain barrier (BBB). J Neurosci 31:365–374
Acknowledgments
This work has been founded by the National R&D Program Grant of the Spanish Ministry of Education and Science (AGL2002-00529 and AGL2005-01561). Noemi Yubero was a postgraduate scholar of the Spanish Research Programme. Juan J. Garrido was a recipient of a “Ramón y Cajal” Grant of the Spanish Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yubero, N., Jiménez-Marín, Á., Lucena, C. et al. Immunohistochemical distribution of the tetraspanin CD9 in normal porcine tissues. Mol Biol Rep 38, 1021–1028 (2011). https://doi.org/10.1007/s11033-010-0198-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0198-8