Abstract
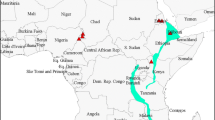
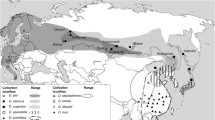
Maruca vitrata Fabricius is a pantropical lepidopteran pest of legumes. Phylogenetic analysis of a mitochondrial cytochrome c oxidase-I gene (cox1) fragment indicates that three Maruca sp. mitochondrial lineages have unique geographic distributions [lineages 1 and 2: Australia, Taiwan, and West Africa (Niger, Nigeria, and Burkina Faso), and lineage 3: Puerto Rico]. The haplotype (T30, T114) is specific to lineages 1&2 and was assayed by NsiI and SacI polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) within population samples; it was not observed in the Puerto Rican samples, but was nearly fixed among samples from West Africa, Australia and Taiwan (85.5–100%). Re-sequencing and phylogenetic analyses of PCR-RFLP defined cox1 haplotypes indicate that nucleotide diversity is highest among samples from West Africa. Phylogenetic reconstruction based upon ribosomal DNA (rDNA) internal transcribed spacer-2 (ITS-2) sequences provided additional evidence for three Maruca sp. clades. These data suggest that multiple unique Maruca species or subspecies are present worldwide, which has implications for the management of this pest species-complex.

Similar content being viewed by others
References
CAB International (2005) Crop protection compendium, 2005 edition. CAB International, Wallingford. www.cabicompendium.org/cpc (selected texts for Maruca vitrata). http://www.runetwork.de/html/fr/articles/document.html?Action=displayDocument&id=8752
Taylor TA (1967) The bionomics of Maruca testululis Gey. (Lepidoptera: Pyralidae), a major pest of cowpeas in Nigeria. J W Afr Sci Assoc 12:111–129
Raheja AI (1974) Report on the insect pests of grain legumes in northern Nigeria. In: 1st IITA grain legume improvement workshop, 1973. International Institute of Tropical Agriculture, Ibadan, Nigeria, pp 295–299
Katayama J, Suzuki I (1984) Seasonal prevalence of pod borers [Ostrinia scapulalis, Maruca testulalis and Matsumuraeses sp.] in azuki-beans and injury caused by larval infestation. Bull Kyoto Prefect Inst Agric 12:27–34
Ke LD, Fang JL, Li ZJ (1985) Bionomics and control of the legume pod-borer Maruca testulalis Geyer. Acta Entomol Sin 28(1):51–59
Sharma HC (1998) Bionomics, host plant resistance, and management of the legume pod borer, Maruca vitrata—a review. Crop Prot 17:373–386
Wolcott GN (1933) The lima bean pod borer caterpillars of Puerto Rico. J Dept Agric P R 17:241–255
Munroe EG (1995) Pyraustinae. In: Heppner JB (ed) Atlas of neotropical Lepidoptera. Checklist: part 2 Hyblaeoidea, Pyraloidea, Tortricoidea. Association for Tropical Lepidoptera, Scientific Publishers, Gainesville
Arodokoun DY, Tamò M, Cloutier C, Adeoti R (2003) The importance of alternative host plants for the annual cycle of the legume pod borer, Maruca vitrata Fabricius (Lepidoptera: Pyralidae). Insect Sci Appl 23:103–113
Rathore YS, Lal SS (1998) Phylogenetic relationship of host plants of Maruca vitrata. Indian J Pulse Res 11(2):152–155
Singh SR, van Emden HF (1979) Insect pests of grain legumes. Annu Rev Entomol 24:255–278
Sharma HC, Saxena KB, Bhagwat VR (1999) The legume pod borer, Maruca vitrata: bionomics and management. Information bulletin 55. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, India
Arodokoun DY, Tamo M, Cloutier C, Brodeur J (2006) Larval parasitoids occurring on Maruca vitrata Fabricius (Lepidoptera: Pyralidae) in Benin, West Africa. Agric Ecosyst Environ 113:320–325
Chinh NT, Dzung DT, Long TD, Tam HM, Ramakrishna A, Johansen C (2000) Legumes in Viet Nam: constraints and opportunities, pp 111–125. In: Gowda CLL, Ramakrishna A, Rupela OP, Wani SP (eds) Legumes in rice-based cropping systems in tropical Asia: constraints and opportunities. ICRISAT, India, 142 pp
Soeun M (2001) Legumes in rice-based cropping systems in Cambodia: constraints and opportunities, pp 4–10. In: Gowda CLL, Ramakrishna A, Rupela OP, Wani SP (eds) Legumes in rice-based cropping systems in tropical Asia: constraints and opportunities. ICRISAT, India, 142 pp
Ulrichs C, Mewis I, Schnitzler WH, Burleigh JR (2001) Parasitoids of the bean podborer, Maruca vitrata F. (Lepidoptera: Pyraustinae), a pest of Vigna sesquipedalis in the Philippine lowlands. Mitteilungen der Deutschen Gesellschaft fur allgemeine und angewandte Entomologie 13(1–6):283–288
Bindra OS (1968) A note on the study of varietal resistance in pulses to different insect pests. In: Second annual workshop on pulse crops. Indian Agricultural Research Institute, New Delhi
Patnaik HP, Samolo AP, Samolo BN (1986) Susceptibility of some early varieties of pigeonpea for pod borers under protected conditions. Legum Res 9:7–10
Rahman MM (1989) Pest complex of flower and pods of pigeonpea and their control through insecticide application. Bang J Sci Res 7(1):27–32
Saxena KB (2000) Pigeonpea. In: Gupta SK (ed) Plant breeding: theory and techniques. Agrobios, Jodhpur, pp 82–112
Ekesi S (1999) Insecticide resistance in field populations of the legume pod borer Maruca vitrata Fabricius in Nigeria. Int J Pest Manag 45:57–59
Ulrichs C, Mewis I, Schnitzler WH, Burleigh JR (2001) Effectivity of synthetic insecticides against Maruca vitrata F and the parasitoid Bassus asper Chou & Sharkey in the Philippines. Mitteilungen der Deutschen Gesellschaft fur allgemeine und angewandte Entomologie 13(1–6):279–282
Kym A (2006) Trangenic crops, EU precaution, and developing countries. Int J Technol Glob 2:65–80
Oparaeke AM (2006) The potential for controlling Maruca vitrata Fab. and Clavigralla tomentosicollis Stal. using different concentrations and spraying schedules of Syzigium aromaticum (L.) Merr and Perr on cowpea plants. J Plant Sci 1:132–137
Chen S, Ravallion M (2004) How have the world’s poorest fared since the early 1980s? World Bank Res Obs 19:141–169
Evenson RE, Gollin D (2003) Assessing the impact of the green revolution, 1960 to 2000. Science 300(5620):758–762
Brader L (1982) Recent trends of insect control in the tropics. Entomol Exp Appl 31:111–120
Saxena KB, Chandrasena GDSN, Hettiarachchi K, Iqbal YB, Fonseka HHD, Jayasekera SJBA (2002) Evaluation of pigeonpea accessions and selected lines for reaction to Maruca. Crop Sci 42(2):615–618
Adekola OF, Oluleye F (2008) Induced tolerance of cowpea mutants to Maruca vitrata (Fabricius) (Lepidoptera : Pyralidae). Afr J Biotechnol 7(7):878–883
Srinivasan R (2008) Susceptibility of legume pod borer (LPB), Maruca vitrata to delta-endotoxins of Bacillus thuringiensis (Bt) in Taiwan. J Invertebr Pathol 97(1):79–81
Murdock LL, Coulibaly O, Higgins TJV, Huesing JE, Ishiyaku MF, Sithole-Niang I (2008) Cowpea: legume grains and forages. In: Kole C, Hall TC (eds) A compendium of transgenic crop plants. Blackwell Publishing, Oxford, pp 23–56
Ba NM, Margam VM, Binso-Dabire CL, Sanon A, McNeil J, Murdock LL, Pittendrigh BR (2009) Seasonal and regional distribution of the cowpea pod borer, Maruca vitrata (Lepidoptera: Crambidae), in Burkina Faso. Int J Trop Insect Sci 29:1–6
Bottenberg H, Tamò M, Arodokoun D, Jackai LEN, Singh BB, Youm O (1997) Population dynamics and migration of cowpea pests in northern Nigeria: implications for integrated pest management. In: Singh BB, Mohan-Raj DR, Dashiell KE, Jackai LEN (eds) Advances in cowpea research. International Institute of Tropical Agriculture and Japan International Center for Agricultural Sciences, Ibadan, pp 271–284
Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W (2004) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA 101:14812–14817
Hebert PDN, Ratnasingham S, deWaard JR (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc Biol Sci 270:S96–S99
Smith MA, Woodley NE, Janzen DH, Hallwachs W, Hebert PDN (2006) DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc Natl Acad Sci USA 103:3657–3662
Smith MA, Wood DM, Janzen DH, Hallwachs W, Hebert PDN (2007) DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc Natl Acad Sci USA 104:4967–4972
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Kumar S, Gadagkar SR (2001) Disparity Index: a simple statistic to measure and test the homogeneity of substitution patterns between molecular sequences. Genetics 158:1321–1327
Saitou N, Nei M (1987) The neighbor-joining method—a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Takahashi K, Nei M (2000) Efficiencies of fast algorithms of phylogenetic Inference under the criteria of maximum parsimony, minimum evolution, and maximum likelihood when a large number of sequences are used. Mol Biol Evol 17:1251–1258
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ratnasingham S, Hebert PDN (2007) BOLD: the barcode of life data system. Mol Ecol Notes 7:355–364. www.barcodinglife.org
Eddy SR (1998) Profile hidden Markov models. Bioinformatics 14:755–763
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JS, White TJ (eds) PCR protocol: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185
Tajima F (1989) Statistical methods to test for nucleotide mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Dwivedi B, Gadagkar SR (2009) The impact of sequence parameter values on phylogenetic accuracy. Biol Med 1:50–62
Kim CG, Hoshizaki S, Huang YP, Tatsuki S (1999) Usefulness of mitochondrial COII gene sequences in examining phylogenetic relationships in the Asian corn borer, Ostrinia furnacalis, and allied species (Lepidoptera: Pyralidae). Appl Entomol Zool 34:405–412
Ohno S, Ishikawa Y, Tatsuki S, Hoshizaki S (2006) Variation in mitochondrial COII gene sequences among two species of Japanese knotweed-boring moths, Ostrinia latipennis and O. ovalipennis (Lepidoptera: Crambidae). Bull Entomol Res 96:243–249
Coates BS, Sumerford DV, Hellmich RL, Lewis LC (2005) Partial mitochondrial genome sequences of Ostrinia nubilalis and Ostrinia furnacalis. Int J Biol Sci 1:13–18
Hoshizaki S, Washimori R, Kubota S, Ohno S, Huang Y, Tatsuki S, Ishikawa Y (2008) Two mitochondrial lineages occur in the Asian corn borer, Ostrinia furnacalis (Lepidoptera:Crambidae), in Japan. Bull Entomol Res 98:519–526
Coates BS, Sumerford DV, Hellmich RL (2004) Geographic and voltinism differentiation among North American Ostrinia nubilalis (European corn borer) mitochondrial cytochrome c oxidase hapoltypes. J Insect Sci 4:35–43
Brower AVZ (2006) Problems with DNA barcodes for species delimitation: ‘ten species’ of Astraptes fulgerator reassessed (Lepidoptera: Hesperiidae). Syst Biodivers 4:127–132
Will KW, Mishler BD, Wheeler QD (2005) The perils of DNA barcoding and the need for integrative taxonomy. Syst Biol 54:844–851
Vignal A, Milan D, San Cristobal M, Eggen A (2002) A review on SNP and other types of molecular markers and their use in animal genetics. Genet Sel Evol 34:275–305
Coates BS, Sumerford DV, Hellmich RL, Lewis LC (2008) Mining an Ostrinia nubilalis midgut expressed sequence tag (EST) library for candidate genes and single nucleotide polymorphisms (SNPs). Insect Mol Biol 17:607–620
Coates BS, Sumerford DV, Miller NJ, Kim KS, Sappington TW, Siegfried BD et al (2009) Comparative performance of single nucleotide polymorphism and microsatellite markers for population genetic analysis. J Hered 100:556–564
Brumfield RT, Beerli P, Nickerson DA, Edwards SV (2003) The utility of single nucleotide polymorphisms in inference of population history. Trends Ecol Evol 18:249–256
Väli U, Einarsson A, Waits L, Ellegren H (2008) To what extent do microsatellite markers reflect genome-wide genetic diversity in natural populations. Mol Ecol 17:3808–3817
Akey JM, Zhang K, Xiong M, Jin L (2003) The effect of single nucleotide polymorphism identification strategies on estimates of linkage disequilibrium. Mol Biol Evol 20:232–242
Morin PA, Luikart G, Wayne RK RK, SNP workshop group (2004) SNPs in ecology, evolution and conservation. Trends Ecol Evol 19:208–216
Feng X, Liu DF, Wang NX, Zhu CD, Jiang GF (2010) The mitochondrial genome of the butterfly Papilio xuthus (Lepidoptera: Papilionidae) and related phylogenetic analyses. Mol Biol Rep. 2010 Mar 8 [Epub ahead of print]. PubMed PMID: 20213506
Hu J, Zhang D, Hao J, Huang D, Cameron S, Zhu C (2009) The complete mitochondrialgenome of the yellow coaster, Acraea issoria (Lepidoptera: Nymphalidae: Heliconiinae: Acraeini): sequence, gene organization and a unique tRNA translocation event. Mol Biol Rep. 2009 Nov 29 [Epub ahead of print]. PubMed. PMID: 20091125
Kim SR, Kim MI, Hong MY, Kim KY, Kang PD, Hwang JS, Han YS, Jin BR, Kim I (2009) The complete mitogenome sequence of the Japanese oak silkmoth, Antheraea yamamai (Lepidoptera: Saturniidae). Mol Biol Rep 36(7):1871–1880. Epub 2008 Nov 2. PubMed PMID: 18979227
Yang L, Wei ZJ, Hong GY, Jiang ST, Wen LP (2009) The complete nucleotide sequence of the mitochondrial genome of Phthonandria atrilineata (Lepidoptera: Geometridae). Mol Biol Rep 36(6):1441–1449. Epub 2008 Aug 12. PubMed PMID: 18696255
Tamò M, Ekesi S, Maniania NK, Cherry A (2003) Biological control, a non-obvious component of integrated pest management for cowpea. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in integrated pest management systems in Africa. CABI Publishing, Wallingford, pp 295–309
Srinivasan R, Tamò M, Ooi PA, Easdown W (2007) IPM for Maruca vitrata on food legumes in Asia and Africa. Biocontrol News Inf 28:34–37
Acknowledgments
This project was supported by a U.S. Agency for International Development (USAID) Bean/Cowpea Collaborative Research Support Program (CRSP) funding to LLM, BRP, IB, CBD, and MFI. VM was supported by a dissertation fellowship from Purdue University. Funding support to BRP, CBD, MB, IB, LLM, and MFI was also partly provided from Dry Grains Pulses Collaborative Research Support Program (CRSP) by the Bureau for Economic Growth, Agriculture, and Trade, U.S. Agency for International Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development or the U.S. government.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2010_182_MOESM1_ESM.doc
Supplemental Fig. S1: Alignment of a 500 bp mitochondrial coxI fragment and corresponding translation obtained from Maruca sp. samples from Taiwan (TAI), Nigeria (NA), and Puerto Rico (PR). Only variable nucleotide positions are indicated, and non-synonymous changes in the peptide sequence are highlighted. The NsiI (ATGCAT) and SacI (GAGCTC) restriction endonuclease recognition sites that are diagnostic for differentiation of Maruca sp. from non-Puerto Rican locations are underlined. (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Margam, V.M., Coates, B.S., Ba, M.N. et al. Geographic distribution of phylogenetically-distinct legume pod borer, Maruca vitrata (Lepidoptera: Pyraloidea: Crambidae). Mol Biol Rep 38, 893–903 (2011). https://doi.org/10.1007/s11033-010-0182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0182-3