Abstract
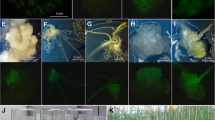
Transgenic doubled haploid rapeseed (Brassica napus L. cvs. Global and PF704) plants were obtained from microspore-derived embryo (MDE) hypocotyls using the microprojectile bombardment. The binary vector pCAMBIA3301 containing the gus and bar genes under control of CaMV 35S promoter was used for bombardment experiments. Transformed plantlets were selected and continuously maintained on selective medium containing 10 mg l−1 phosphinothricin (PPT) and transgenic plants were obtained by selecting transformed secondary embryos. The presence, copy numbers and expression of the transgenes were confirmed by PCR, Southern blot, RT-PCR and histochemical GUS analyses. In progeny test, three out of four primary transformants for bar gene produced homozygous lines. The ploidy level of transformed plants was confirmed by flow cytometery analysis before colchicine treatment. All of the regenerated plants were haploid except one that was spontaneous diploid. High frequency of transgenic doubled haploid rapeseeds (about 15.55% for bar gene and 11.11% for gus gene) were considerably produced after colchicines treatment of the haploid plantlets. This result show a remarkable increase in production of transgenic doubled haploid rapeseed plants compared to previous studies.





Similar content being viewed by others
Abbreviations
- MDE:
-
Microspore-derived embryo
- PPT:
-
Phosphinothricin
References
Fry J, Barnason A, Horsch RB (1987) Transformation of Brassica napus with Agrobacterium tumefaciens based vectors. Plant Cell Rep 6:321–325
Pua EC, Mehra-Palta A, Nagy F, Chua NH (1987) Transgenic plants of Brassica napus L. Biotechnology 5:815–817
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transfonnation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8:238–242
Stewart CN Jr, Adang MJ, All JN, Raymer PL, Ramachandran S, Parrott WA (1996) Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis cryIAc gene. Plant Physiol 112:115–120
Cardoza V, Stewart CN (2003) Increased Agrobacterium-mediated transfonnation and rooting efficiencies in canola (Brassica napus L.) from hypocotyls segment explants. Plant Cell Rep 21:599–604
Herve C, Rouan D, Guerche P, Montane MH, Yot P (1993) Molecular analysis of transgenic rapeseed plants obtained by direct transfer of two separate plasmids containing, respectively, the cauliflower mosaic virus coat protein gene and a selectable marker gene. Plant Sci 91:181–193
Jones-Villeneuve E, Huang B, Prudhomme I, Bird S, Kemble R, Hattori J, Miki B (1995) Assessment of microinjection for introducing DNA into uninuclear microspores of rapeseed. Plant Cell Tissue Organ Cult 40:97–100
Dormann M, Wang HM, Datla N, Ferrie AMR, Keller WA, Oelck MM (1995) Transformation of freshly isolated Brassica microspores and regeneration of fertile homozygous plants. In: Proc 9th Int Rapeseed Congr, Cambridge, UK, 4–7 July, Groupe Consultating International de Recherche sur le Colza, pp 816–818
Dormann M, Datla N, Hayden A, Puttick D, Quandt J (1998) Non-destructive screening of haploid embryos for glufosinate ammonium resistance four weeks after microspore transformation in Brassica. Acta Hortic 459:191–197
Fukuoka H, Ogawa T, Matsuoka M, Ohkawa Y, Yano H (1998) Direct gene delivery into isolated microspores of rapeseed (Brassica napus L.) and the production of fertile transgenic plants. Plant Cell Rep 17:323–328
Nehlian L, Möllers C, Bergman P, Glimelius K (2000) Transient β-gus and gfp gene expression and viability analysis of microprojectile bombarded microspores of Brassica napus L. J Plant Physiol 156:175–183
Aulinger IE (2002) Combination of in vitro androgenesis and biolistic transformation: an approach for breeding transgenic Maize (Zea mays L.) lines. PhD thesis, Swiss Federal Institute of Technology (ETH), Zurich, Switzerland
Stöger E, Fink C, Pfosser M, Heberle-Bors E (1995) Plant transformation by particle bombardment of embryogenic pollen. Plant Cell Rep 14:273–278
Jähne A, Becker D, Brettschneider R, Lörz H (1994) Regeneration of transgenic, microspore derived, fertile barley. Theor Appl Genet 89:525–533
Yao QA, Simion E, William M, Krochko J, Kasha KJ (1997) Biolistic transformation of haploid isolated microspores of barley (Hordeum vulgare L.). Genome 40:570–581
Carlson AR, Letarte J, Chen J, Kasha KJ (2001) Visual screening of microspore-derivedtransgenic barley (Hordeum vulgare L.) with green-fluorescent protein. Plant Cell Rep 20:331–337
Chaïr H, Legavre T, Guiderdoni E (1996) Transformation of haploid. microspore-derived cell suspension protoplasts of rice (Oryza sativa L.). Plant Cell Rep 15:766–770
Mentewab A, Letellier V, Marque C, Sarrafi A (1999) Use of anthocyanin biosynthesis stimulatory genes as markers for the genetic transformation of haploid embryos and isolated microspores in wheat. Cereal Res Commun 27:17–24
Folling L, Olesen A (2001) Transformation of wheat (Triticum aestivum L.) microspore-derived callus and microspores by particle bombardment. Plant Cell Rep 20:629–636
Liu W (2004) Transformation of microspores for generating doubled haploid transgenic Wheat (Triticum aestivum L.). PhD thesis, Washington State University
Jardinaud MF, Souvré A, Alibert G, Beckert M (1995) UidA gene transfer and expression in maize microspores using the biolistic method. Protoplasma 187:138–143
Jardinaud MF, Souvré A, Beckert M, Alibert G (1995) Optimization of DNA transfer and transient β-glucuronidase expression in electroporated maize (Zea mays L.) microspores. Plant Cell Rep 15:55–58
Aulinger IE, Peter SO, Schmid JE, Stamp P (2003) Gametic embryos of maize as a target for biolistic transformation: comparison to immature zygotic embryos. Plant Cell Rep 21:585–591
Abdollahi MR, Corral-Martınez P, Mousavi A, Salmanian AH, Moieni A, Seguí-Simarro JM (2009) An efficient method for transformation of pre-androgenic, isolated Brassica napus microspores involving microprojectile bombardment and Agrobacterium-mediated transformation. Acta Physiol Plant. doi:10.1007/s11738-009-0365-5
Abdollahi MR, Moieni A, Mousavi A, Salmanian AH (2009) Secondary embryogenesis and transient expression of the β-glucuronidase gene in hypocotyls of rapeseed (Brassica napus L.) microspore-derived embryos. Biol Plant 53:573–577
Abdollahi MR, Moieni A, Mousavi A, Salmanian AH, Jalali Javaran M, Majdi M (2007) Effect of integrated bombardment and Agrobacterium transformation system on transient GUS expression in hypocotyls of rapeseed (Brassica napus L. cv. PF704) microspore-derived embryos. Pak J Biol Sci 10:3141–3145
Nehlin L, Möllers C, Glimelius K (1995) Induction of secondary embryogenesis in microsporederived embryos of Brassica napus L. Plant Sci 111:219–227
Sangwan RS, Dubois F, Ducrocq C, Bourgeois Y, Vilcot B, Pawlicki N, Sangwan-Norreel BS (1995) The embryo as a tool for genetic engineering in higher plants. In: Teeri M, Cella R, Falavigna A (eds) Current issues in plant molecular and cellular biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 271–277
Fletcher R, Coventry J, Kott LS (1998) Double haploid technology for spring and winter Brassica napus. Technical Bulletin. Department of Plant Agriculture, University of Guelph; OAC Publication, Canada
Chen JL, Beversdorf WD (1994) A combined use of microprojectile bombardment and DNA imbibition enhances transformation frequency of canola. Theor Appl Genet 88:187–192
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 10 8(19):4321–4325
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Habour Laboratory Press, New York
Jonoubi P, Mousavi A, Majd A, Salmanian AH, Jalali Javaran M, Daneshian J (2005) Efficient regeneration of Brassica napus L. hypocotyls and genetic transformation by Agrobacterium tumefaciens. Biol Plant 49:175–180
Sanford JC, Smith FD, Russel JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–509
Yao Q, Cong L, He GY, Chang JL, Li KX, Yang GX (2007) Optimization of wheat co-transformation procedure with gene cassettes resulted in an improvement in transformation frequency. Mol Biol Rep 34:61–67
Sharry S, Ponce JLC, Estrella LH, Cano RMR, Lede S, Abedini W (2006) An alternative pathway for plant in vitro regeneration of chinaberry—tree Melia azedarach L. derived from the induction of somatic embryogenesis. Electron J Biotechnol 9:187–194
Li B, Wolyn D (1996) Temperature and genotype affect asparagus somatic embryogenesis. In Vitro Cell Dev Biol Plant 32:136–139
Coventry J, Kott L, Beversdorf WD (1988) Manual for microspore culture technique for Brassica napus. Technical Bulletin, OAC Publication 0489. Department of Crop Science, University of Guelph, Guelph
Puigderrajols P, Mir G, Molinas M (2001) Ultrastructure of early secondary embryogenesis by multicellular and unicellular pathways in Cork Oak (Quercus suber L.). Ann Bot 87:179–189
Palmer CE, Oelck M (1992) The relationship of phosphinothricin to growth and metabolism in cell cultures of Brassica napus L. Plant Physiol 141:105–110
Custers JBM, Snepvangers SCHJ, Jansen HJ, Zhang L, van Lookeren Campagne MM (1999) The 35S-CaMV promoter is silent during early embryogenesis but activated during nonembryogenic sporophytic development in microspore culture. Protoplasma 208:257–264
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
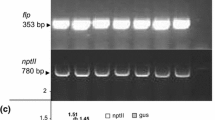
Molecular characterization of putative bar transformants via PCR. MW molecular weight marker 1 kb DNA ladder (Fermentas); C+, positive control (pCAMBIA3301); C−, negative control (without DNA template); NT, non-transformed plant control; T1−14, putative transformants. The expected size of the PCR product is indicated with arrow (TIFF 811 kb)
Figure 2
Molecular characterization of putative gus transformants via PCR. MW, molecular weight marker 1 kb DNA ladder (Fermentas); C+, positive control (pCAMBIA3301); C−, negative control (without DNA template); NT, non-transformed plant control; T3 and T5–T13, putative transformants, The expected size of the PCR product is indicated with arrow (TIFF 1084 kb)
Figure 3
RT-PCR results of the rapeseed haploid transgenic plants for gus, bar and 18S genes. MW, molecular weight markers 1 kb DNA ladder (Fermentas); C−, negative control (water template); NT, negative control (RNA of a non-transgenic plant); T1 and T2, RNA of two transgenic lines. C+, positive control (TIFF 988 kb)
Rights and permissions
About this article
Cite this article
Abdollahi, M.R., Moieni, A., Mousavi, A. et al. High frequency production of rapeseed transgenic plants via combination of microprojectile bombardment and secondary embryogenesis of microspore-derived embryos. Mol Biol Rep 38, 711–719 (2011). https://doi.org/10.1007/s11033-010-0158-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0158-3