Abstract
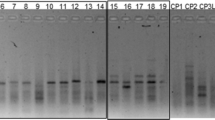
Genetic structure of four populations of Metapenaeus affinis from Maharashtra, Orissa, Kerala and Tamil Nadu in India was studied using RAPD markers. Five selective primers provided distinct and consistent RAPD profiles in all the four populations. The bands in the range 225–1,900 bp were scored for consistent results. The RAPD profiles generated by all the five primers revealed varying degrees of polymorphism, ranging from 25.00% (primer E-03) to 65.00% (primer E-06). Nei’s (Nei M, Natl Acad Sci Proc USA 70:3321–3323, 1973) genetic diversity (h) among the four populations varied from 0.2565 ± 0.2146 (Orissa population) to 0.3576 ± 0.1897 (Maharashtra population).




Similar content being viewed by others
References
Edwards HM (1837) Synopsis of biological data on the penaeid prawn metapenaeus affinis. FAO fisheries synopsis no. 98
Lakra WS, Goswami M, Gopalakrishnan A (2009) Molecular identification and phylogenetic relationships of seven Indian Sciaenids (Pisces: Perciformes, Sciaenidae) based on 16S rRNA and cytochrome c oxidase subunit I mitochondrial genes. Mol Biol Rep. 2009 May; 36(5):831–839. Epub 2008 Apr 16. PubMed PMID: 18415704
Persis M, Chandra Sekhar Reddy A, Rao LM, Khedkar GD, Ravinder K, Nasruddin K (2009) COI (cytochrome oxidase-I) sequence based studies of Carangid fishes from Kakinada coast, India. Mol Biol Rep. 2009 Sep; 36(7):1733–1740. Epub 2008 Oct 12. PubMed PMID: 18850326
Munasinghe H, Burridge C, Austin C (2004) The systematics of freshwater crayfish of the genus Cherax Erichson (Decapoda:Parastacidae) in eastern Australia re-examined using nucleotide sequences from 12S rRNA and 16S rRNA genes. Invertebr Syst Aust J Sci Res 18:215–225
Welsh J, McClelland M (1990) Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res 18:7213–7218
Williams JGK, Kubilik AR, Livak KL, Rafaski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Bardakci F, Skibinski DOF (1994) Applications of the RAPD technique in tilapia fish: species and subspecies identification. Heredity 73:117–123
Garcia DK, Benzie JAH (1995) RAPD markers of potential use in penaeid prawn (Penaeus monodon) breeding programs. Aquaculture 130:137–144
Naish KA, Warren M, Bardakci F, Skibinski DOF, Carvalho GR, Mair GC (1995) Multilocus DNA fingerprinting and RAPD reveal similar genetic relationships between strains of Oreochromis niloticus (Pisces: Cichlidae). Mol Ecol 4:271–274
Dahle G, Rahman M, Eriksen AG (1997) RAPD fingerprinting used for discriminating among three populations of Hilsa shad (Tenualosa ilisha). Fish Res 32:263–269
Lakra WS, Goswami M, Mohindra V, Lal KK, Punia P (2007) Molecular identification of five Indian sciaenids (Pisces: Perciformes, Sciaenidae) using RAPD markers. Hydrobiologia 583:359–363
Mishra PS, Chaudhari A, Krishna G, Kumar D, Lakra WS (2009) Genetic diversity in Metapenaeus dobsonii using RAPD analysis. Biochem Genet 47:421–426
Saini A, Dua A, Mohindra V, Lakra WS (2010) Molecular discrimination of six species of Bagrid catfishes from Indus river system using randomly amplified polymorphic DNA markers. Mol Biol Rep. 2010 Feb 2. [Epub ahead of print] PubMed PMID: 20127179
Abdul Muneer PM, Gopalakrishnan A, Musammilu KK, Mohindra V, Lal KK, Basheer VS, Lakra WS (2009) Genetic variation and population structure of endemic yellow catfish, Horabagrus brachysoma (Bagridae) among three populations of Western Ghat region using RAPD and microsatellite markers. Mol Biol Rep. 2009 Sep;36(7):1779–1791. Epub 2008 Nov 2. PubMed PMID: 18979230
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Yeh FC, Yang RC, Boyle T (1999) Popgene version 1.31: Microsoft windows-based freeware for population genetic analysis
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco
Miller MP (1997) Tools for Population Genetic Analyses (TFPGA), version 1.3. A windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author
Nei M (1973) Analysis of genetic diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Carvalho GR, Hauser L (1994) Molecular genetics and the stock concept in fisheries. Rev Fish Biol Fish 4(3): 326–350. ISSN: 0960-3166
Lester LJ (1983) Developing a selective breeding program for penaeid shrimp mariculture. Aquaculture 33:41–50
Dimmock A, Williamson I, Mather P (2002) Environmental effects on morphology of mature male Macrobrachium australiense (Decapoda: Palaemonidae), European Aquaculture Society, Belgium. Special publication no. 32:208–209
Avise JC (1994) Molecular markers, natural history, and evolution. Chapman and Hall, New York
Garcia DK, Faggart MA, Rhodes L, Alcivar-Warren AA, Wyban JA, Carr WH, Sweeney JN, Ebert KM (1994) Genetic diversity of cultured Penaeus vannamei shrimp using three molecular genetic techniques. Mol Mar Biol Biotechnol 3:270–280
Klinbunga S, Siludjai D, Wuthijinda W, Tassanakajon A, Jarayabhand A, Menasveta P (2001) Genetic heterogeneity of the giant tiger shrimp (Penaeus monodon) in Thailand revealed by RAPD and mtDNA-RFLP analyses. Mar Biotechnol 3:428–438
Tassanakajon A, Pongsomboon S, Rimphanitchayakit V, Jarayabhand P, Boonsaeng V (1998) Genetic structure in wild populations of black tiger shrimp (Penaeus monodon) using randomly amplified polymorphic DNA analysis. J Mar Biotechnol 6:249–254
Liu Z, Jarret RL, Duncan RR, Kresovich S (1994) Genetic relationships and variation among ecotypes of seashore paspalum (Paspalum vaginatum) determined by random polymorphic DNA markers. Genome 37:1011–1017
Acknowledgements
The authors are grateful to Dr. Mangala Rai, Director General, Indian Council of Agricultural Research and Dr. S. Ayyappan, Dy. Director General (Fisheries) for the encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakra, W.S., Goswami, M., Singh, A. et al. Genetic characterization of Metapenaeus affinis (H. M. Edwards, 1837) using RAPD markers. Mol Biol Rep 37, 3757–3761 (2010). https://doi.org/10.1007/s11033-010-0029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0029-y