Abstract
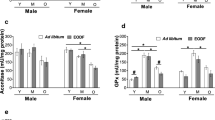
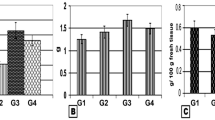
Aging, a multifactorial process of enormous complexity is characterised by physio-chemical and biological aspects of cellular functions. It is closely associated with changes in metabolism of various biological molecules in the system. In the present study, we have investigated the effect of deprenyl on cerebellum during ageing process in male Wistar rats with respect to the changes in levels of protein, glycoproteins and amino acids in experimental rats of three age groups (6, 12 and 18 months old). Intraperitoneal administration of liquid deprenyl (2 mg/kg body weight/day for a period of 15 days i.p., significantly P < 0.05) attenuated age-associated alterations in the levels of amino acids (taurine, aspartate, glutamate, arginine, hydroxy proline and homocysteine), protein content and glycoprotein components (hexose and hexosamine) in the rat cerebellum. The results of the present investigation indicate that the protective effect of deprenyl is probably related to its ability to strengthen the neuronal membrane by its membrane stabilizing action or to a counteraction of free radicals by its antioxidant property.







Similar content being viewed by others
References
Wei YH, Pang CY, Lee HC, Lu CY (1998) Roles of mitochondrial DNA mutation and oxidative damage in human aging. Curr Sci 74:887–893
Toescu EC, Myronova N, Verkhratsky A (2000) Age-related structural and functional changes of brain mitochondria. Cell Calcium 28:329–338
Kowald A (2002) Life span does not measure ageing. Biogerontology 3:187–190
Kirkwood TBL, Adams C, Gibbons L, Hewitt C (1996) Cell maintenance and stress response in ageing and longevity. In: Rattan S, Toussaint O (eds) Molecular gerontology: research status and strategies. Plenum, New York, pp 193–200
Bharath S, Anderson JK (2004) Catecholamine and protein deposition in Parkinson’s and Alzheimer’s disease: old medicine, new targets. Rejuvenation Res 7:92–94
Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Gono S (2002) Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol 37:1423–1430
Bowen J, Evangelista AM (2002) Brain cell damage from amino acid isolates: a primary concern from aspartame-based products and artificial sweetening agents—nutrasweet equal “sugar free” neotame. http://Wnho.net/aspartame_brain_damage.htm. Retrieved May 11, 2007
James TJ, Sharma SP, Gupta SK, Patro IK (1992) ‘Dark’ cell formation under protein malnutrition: process of conversion and concept of ‘semi-dark’ type Purkinje cells. Indian J Exp Biol 30:470
Vohra BPS, James TJ, Sharma SP, Kansal VK, Chudhary A, Gupta SK (2002) Dark neurons in the ageing cerebellum: their mode of formation and effect of Maharishi Amrit Kalash. Biogerontology 3:347–354
Knoll J, Dallo J, Yen TT (1989) Striatal dopamine, sexual activity and lifespan longevity of rats treated with (-) deprenyl. Life Sci 45:525–531
Magyar K, Palfi M, Tabi T, Kalasz H, Szende B, Szoko E (2004) Pharmacological aspects of (-)-deprenyl. Curr Med Chem 11:2017–2031
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD (1997) Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282
Ishida Y, Fugita T, Asai K (1981) New detection and separation method for aminoacid by high performance liquid chromatography. J Chromatogr 204:143–148
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Niebes P (1972) Determination of enzymes and degradation of the products of glycosylamino-glycan metabolism in healthy and various subjects. Clin Chim Acta 42:399–408
Wagner WD (1979) More sensitive assay discriminating galactosamine and glycosamine in mixtures. Anal Biochem 94:394–396
Olive MF (2002) Interactions between taurine and ethanol in the central nervous system. Amino Acids 23:345–357
Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: An Update. Neurochem Res 30:1615–1621
Banay-Schwartz M, Lajtha A, Palkovits M (1989) Changes with aging in the levels of amino acids in rat CNS structural elements II. Taurine and small neutral amino acids. Neurochem Res 14:563–570
El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328
Philip EC, Matthew TG, Phillip JS, Alexander RJ, Hongjie Y, Amanda LJ, James PS, Stephen FT, David JAW (2005) Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Mol Pharmacol 67:1470–1484
Song D, O’Regan MH, Phillis JW (1996) Release of the excitotoxic amino acids, glutamate and aspartate, from the isolated ischemic/anoxic rat heart. Neurosci Lett 220:1–4
De Marchi U, Pietrangeli P, Mondovi B, Toninello A (2003) Deprenyl as an inhibitor of menadione-induced permeability transition in liver mitochondria. Biochem Pharmacol 66:1749–1754
Svetlana MM, Simo SO, Pirjo S (2006) Taurine attenuates d-[3H] aspartate release evoked by depolarization in ischemic corticostriatal slices. Brain Res 1099:64–72
Kuiper MA, Visser JJ, Bergmans PL, Scheltens P, Wolters EC (1994) Decreased cerebrospinal fluid nitrate levels in Parkinson’s disease, Alzheimer’s disease and multiple system atrophy patients. J Neurol Sci 121:46–49
Mollace V, Rodino P, Massoud R, Rotiroti D, Nistico G (1995) Age-dependent changes of NO synthase activity in the rat brain. Biochem Biophys Res Commun 215:3822–3827
Eva S, Esther M-L, Ana C, Marta S, Raquel H, Juan CL-R, Maria LDM, Francisco JE, Santos B, Juan AP, Jose R, Maria AP (2002) Age-related changes of the nitric oxide system in the rat brain. Brain Res 956:385–392
Dominique L, Mehrnaz J-T, Gaelle L, Bruno P, Dominique B-R, Michel P, Isabelle M (2005) Lack of iNOS induction in a severe model of transient focal cerebral ischemia in rats. Exp Neurol 195:218–228
Thomas T, McLendon C, Thomas G (1998) l-Deprenyl: nitric oxide production and dilation of cerebral blood vessels [Ageing]. Neuroreport 9:2595–2600
Arstall MA, Sawyer DB, Fukazawa R, Kelly RA (1999) Cytokine-mediated apoptosis in cardiac myocytes: the role of inducible nitric oxide synthase induction and peroxynitrite generation. Circ Res 85:829–840
Arakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, Timasheff SN (2007) Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem 127:1–8
Kalaria RN, Pax AB (1995) Increased collagen content of cerebral microvessels in Alzheimer’s disease. Brain Res 705:349–352
Czerniczyniec A, Bustamante J, Lores-Arnaiz S (2007) Improvement of mouse brain mitochondrial function after deprenyl treatment. Neuroscience 144:685–693
Tatton WG, Ju WYL, Holland DP, Tai C, Kwan M (2002) Deprenyl reduces PC12 cell apoptosis by inducing new protein synthesis. J Neurochem 63:1572–1575
Tatton WG, Chalmers-Redman RM, Ju WJ, Mammen M, Carlile GW, Pong AW, Tatton NA (2002) Propargylamines induce antiapoptotic new protein synthesis in serum- and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther 301:753–764
Toshifumi M, Miyako N, Masahiro M, Takefumi Y, Hiroshi Y, Haruko T, Mari O, Naoki T, Sachio M, Susumu H, Yo-ichi Y, Takashi S, Koh I, Katsutoshi F, Hiroyuki A (2005) Plasma homocysteine and risk of coexisting silent brain infarction in Alzheimer’s disease. Neurodegener Dis 2:299–304
den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MMB (2003) Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126:170–175
Picerno I, Chirico C, Condello S, Visalli G, Ferlazzo N, Gorgone G, Caccamo D, Ientile R (2006) Homocysteine induces DNA damage and alterations in proliferative capacity of T-lymphocytes: a model for immunosenescence? Biogerontology 8:111–119
Thakur MK, Prasad S (1991) Analysis of age-associated alteration in the synthesis of HMG nonhistone proteins of the rat liver. Mol Biol Rep 15(1):19–24
Bardag-Gorce F, Farout L, Veyrat-Durebex C, Briand Y, Briand M (1999) Changes in 20S proteasome activity during ageing of the LOU rat. Mol Biol Rep 26(1–2):89–93
Supakar PC, Kanungo MS (1984) Conformational changes in the chromatin of the brain of developing rats and its modulation by zinc chloride. Mol Biol Rep 9(4):253–257
Das BR, Kanungo MS (1986) Developmental changes in the chromatin of the brain of the rat: analysis by nick-translation. Mol Biol Rep 11(4):195–197
Conconi M, Friguet B (1997) Proteasome inactivation upon aging and on oxidation-effect of HSP 90. Mol Biol Rep 24(1–2):45–50
Pathak RU, Kanungo MS (2007) Subtractive differential display: a modified differential display technique for isolating differentially expressed genes. Mol Biol Rep 34(1):41–46
Chaurasia P, Thakur MK (1998) Nucleosome positioning and periodicity of satellite DNA in the liver of aging rats. Nucleosome positioning and periodicity of satellite DNA. Mol Biol Rep 25(1):63–69
Mattson MPG (2003) Diet interactions in brain aging and neurodegenerative disorders. Ann Med Sci 139:441–444
Ward WF (2000) The relentless effects of the aging process on protein turnover. Biogerontology 1:195–199
Carrard G, Bulteau AL, Petropoulos I, Friguet B (2002) Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol 34:1461–1474
Chondrogianni N, Fragoulis EG, Gonos S (2002) Protein degradation during aging: the lysosome-, the calpain- and the proteasome-dependent cellular proteolytic systems. Biogerontology 3:121–123
Hipkiss AR (2006) Accumulation of altered proteins and aging: causes and effects. Exp Gerontol 41:464–473
Tatton WG (1996) Deprenyl reduce neuronal apoptosis and facilitates neuronal outgrowth by altering protein synthesis without inhibiting monoamine oxidase. J Neural Transm Suppl 48:45–59
Altman J, Bayer SA (1997) Development of the cerebellar system. CRC Press, New York
Patro N, Gupta P, Patro IK (2002) Lipofuscin in cell ageing and cell death. J Gerontol 16:45–68
Sturrock RR (1989) Age related changes in Purkinje cell number in the cerebellar nodulus of the mouse. J Hirnforsch 30:757–760
Amenta F, Bongrani S, Cadel S, Ferrante F, Valsecchi B, Zeng YC (1994) Influence of treatment with deprenyl on the structure of the cerebellar cortex of aged rats. Mech Ageing Dev 75:157–167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subramanian, M.V., James, T.J. Supplementation of deprenyl attenuates age associated alterations in rat cerebellum. Mol Biol Rep 37, 3653–3661 (2010). https://doi.org/10.1007/s11033-010-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0017-2