Abstract
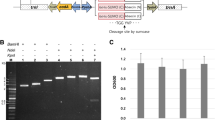
Attacin, a 20 kDa antibacterial peptide, plays an important role in immunity. To understand this gene better, gene cloning, expression and biological activity detection of Attacin A was carried out in present study. The full-length open reading frame (ORF) coding for Attacin A gene was generated using RT-PCR which takes total RNA extracted from Drosophila as the template. The gene was inserted directionally into the prokaryotic expression vector pET-32a (+). The resulting recombinant plasmid was transformed into E. coli Rosetta. SDS–PAGE was carried out to detect the expression product which was induced by IPTG. The antimicrobial activity and hemolysis activity were tested in vitro after purification. Agarose gel electrophoresis indicated that the complete ORF of Attacin A gene has been cloned successfully from Drosophila stimulated by E. coli which includes 666 bp and encodes 221 AA. The gene encoding mature Attacin A protein was amplified by PCR from the recombinant plasmid containing Attacin A, which includes 570 bp in all. SDS–PAGE analysis demonstrated that the fusion protein expressed was approximately 39.2 kDa. Biological activities detection showed that this peptide exhibited certain antibacterial activity to several G− bacteria, as well as minor hemolysis activity for porcine red blood cells. In conclusion, Attacin A gene was cloned and expressed successfully. It was the basis for further study of Attacin.




Similar content being viewed by others
References
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Hultmark D, Engström A, Andersson K, Steiner H, Bennich H, Boman HG (1983) Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO 2:571–576
Carlsson A, Engström P, Palva ET, Bennich H (1991) Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect Immun 59:3040–3045
Sun SC, Lindström I, Lee JY, Faye I (1991) Structure and expression of the Attacin genes in Hyalophora cecropia. Eur J Biochem 196:247–254
Hu YJ, Aksoy S (2002) In vitro expression of tsetse Attacin and its activity against Trypanosoma brucei. Am J Trop Med Hyg 67:371
Xu JH, Zhu JY, Jin XB, Xu QY (2007) Cloning and expression of antibacterial peptide Attacin gene isolated from Musca Domestica Larvae and its biological activities. J Med Mol Biol 4:20–26
Carlsson A, Nyström T, Cock DH, Bennich H (1998) Attacin-an insect immune protein-binds LPS and triggers the specific inhibition of bacterial outer-membrane synthesis. Microbiol 144:2179–2188
Geng H, An CJ, Hao YJ, Li DS, Du RQ (2004) Molecular cloning and expression of Attacin from housefly (Musca domestica). Acta Genet Sin 31:1344–1350
Sugiyama M, Kuniyoshi H, Kotani E, Taniai K, Kadono-Okuda K, Kato Y, Yamamoto M, Shimabukuro M, Chowdhury S, Xu J, Choi SK, Kataoka H, Suzuki A, Yamakawa M (1995) Characterization of a Bombyx mori cDNA encoding a novel member of the Attacin family of insect antibacterial proteins. Insect Biochem Mol Biol 25:385–392
Zhang B, Wang LM, Ye B, Li SY, Zhao ZJ, Fan Q (2006) Cloning and analysis of cDNA of the Attacin gene from the Chinese oak silkworm, Antheraea pernyi, using RLM-RACE method. Canye Kexue 32:333–339
Kang DW, Lundström A, Steiner H (1996) Trichoplusia ni attacin A, a differentially displayed insect coding for an antibacterial protein. Gene 174:245–249
Taniai K, Ishii T, Sugiyama M, Miyanoshita A, Yamakawa M (1996) Nucleotide sequence of 5′-upstream region and expression of the a silkworm gene encoding a new member of the Attacin family. Biochem Biophys Res Commun 220:594–599
Wingfield PT (2005) Preparation of soluble proteins from Escherichia coli. Curr Protoc Pro Sci Chapter 6: Unit 6.2
Hultmark D, Steiner H, Rasmuson T, Boman HG (1980) Insect immunity: purification and properties of the inducible bacterial proteins from hemolymph of immunized pupae of Hyalophora cecropin. Eur J Biochem 106:7–16
Yang ST, Shin SY, Hahm KS, Kim JI (2006) Design of perfectly symmetric Trp-rich peptides with potent and broad-spectrum antimicrobial activities. Int J Antimicro Age 27:325–330
Bignami GS (1993) A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon 31:817–820
Skosyrev VS, Kulesskiy EA, Yakhnin AV, Temirov YV, Vinokurov LW (2003) Expression of the recombinant antibacterial peptide sarcotoxin IA in Escherichia coli cells. Protein Expr Purif 28:350–356
Liu HR, Li GD, Wu HY, Huang JJ, Wang SL, Huang QS (2008) Fusion expression of antimicrobial peptide GK1 in Escherichia coli. Chin J Biotech 24:21–26
Pazgier M, Lubkowski J (2006) Expression and purification of recombinant human α-defensins in Escherichia coli. Protein Expr Purif 49:1–8
Palmer I, Wingfield PT (2004) Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli. Curr Protoc Pro Sci Chapter 6: Unit 6.3
Mukhija R, Rupa P, Pillai D, Garg LC (1995) High level production and single purification of biologically active human growth hormone in E. coli. Gene 165:303–306
Goswami PP, Rupa P, Parihar NS, Garg LC (1996) Molecular cloning of Clostridium perfringens epsilon-toxin gene and its high level expression in E. coli. Biochem Biophys Res Commun 226:735–740
Inouye S, Sahara Y (2008) Soluble protein expression in E. coli cells using IgG-binging domain of protein A as a solubilizing partner in the cold induced system. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2008.08.149
Kwon YM, Kim HJ, Kim YI, Kang YJ, Lee IH, Jin BR, Han YS, Cheon HM, Ha NG, Seo SJ (2008) Comparative analysis of two attacin genes from Hyphantria cunea. Comp Biochem Physiol B Biochem Mol Biol 151:213–220
Lai R, Zheng YT, Shen JH, Liu H, Lee WH, Tang SZ, Zhang Y (2002) Antimicrobial peptides from skin secretions of Chinese red belly toad Bombina maxima. Peptides 23:427–435
Acknowledgments
This work was granted by Feed Biotechnology Project of Sichuan Province of China with grant No. 2007Z06-050 and Program for Changjiang Scholars and Innovative Research Team in University with grant. No. IRTO 555-5, China Ministry of Education. We would like to express our appreciation to Dr. Guo-Quan Han for his expert technical assistance to this study. The authors are also grateful to X. F. Liu for providing the agarose beads used for protein purification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, LN., Yu, B., Han, GQ. et al. Molecular cloning, expression in Escherichia coli of Attacin A gene from Drosophila and detection of biological activity. Mol Biol Rep 37, 2463–2469 (2010). https://doi.org/10.1007/s11033-009-9758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9758-1