Abstract
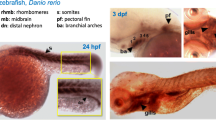
The notochord, a defining characteristic of the chordate embryo is a critical midline structure required for axial skeletal formation in vertebrates, and acts as a signaling center throughout embryonic development. We utilized the digital differential display program of the National Center for Biotechnology Information, and identified a contig of expressed sequence tags (no. Dr. 83747) from the zebrafish ovary library in Genbank. Full-length cDNA of the identified gene was cloned by 5′- and 3′- RACE, and the resulting sequence was confirmed by polymerase chain reaction and sequencing. The cDNA clone contains 2,505 base pairs and encodes a novel protein of 707 amino acids that shares no significant homology with any known proteins. This gene was expressed in mature oocytes and at the one-cell stage, and persisted until the 5th day of development, as determined by RT-PCR. Transcripts were detected by whole-mount RNA in situ hybridization from the two-cell stage to 72 h of embryonic development. This gene was uniformly distributed from the cleavage stage up to the blastula stage. During early gastrulation, it was present in the dorsal region, and became restricted to the notochord and pectoral fin at 48 and 72 h of embryonic development. Based on its abundance in the notochord, we hypothesized that the novel gene may play an important role in notochord development in zebrafish; we named this gene, zebrafish notochord-related gene, or znrg.






Similar content being viewed by others
References
Fleming A, Keynes R, Tannahill D (2004) A central role for the notochord in vertebral patterning. Development 131:873–880
Hogan B et al (1994) Early mouse development. In: Manipulating the mouse embryo, Cold Spring Harbor Laboratory Press, NY, pp 63–81
Scott A, Stemple DL (2005) Zebrafish notochordal basement membrane: signaling and structure. Curr Top Dev Biol 65:229–253
Christ B, Huang R, Scaal M (2004) Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 208:333–350
Cleaver O, Krieg PA (2001) Notochord patterning of the endoderm. Dev Biol 234:1–12
Fouquet B et al (1997) Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol 183:37–48
Goldstein AM, Fishman MC (1998) Notochord regulates cardiac lineage in zebrafish embryos. Dev Biol 201:247–252
Stemple DL (2005) Structure and function of the notochord: an essential organ for chordate development. Development 132:2503–2512
Barresi MJ, Stickney HL, Devoto SH (2000) The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development 127:2189–2199
Cleaver O, Seufert DW, Krieg PA (2000) Endoderm patterning by the notochord: development of the hypochord in Xenopus. Development 127:869–879
Lefebvre V, Li P, de Crombrugghe B (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J 17:5718–5733
Ng LJ et al (1997) SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol 183:108–121
Schulte-Merker S et al (1994) no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120:1009–1015
Talbot WS et al (1995) A homeobox gene essential for zebrafish notochord development. Nature 378:150–157
Parsons MJ et al (2002) Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129:3137–3146
Pagnon-Minot A et al (2008) Collagen XV, a novel factor in zebrafish notochord differentiation and muscle development. Dev Biol 316:21–35
Pollard SM et al (2006) Essential and overlapping roles for laminin alpha chains in notochord and blood vessel formation. Dev Biol 289:64–76
Westerfield M (1995) The zebrafish book. Eugene Press
Kimmel CB et al (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Selman K et al (1993) Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol 218:203–224
Dijkman HBPM et al (1995) RNA in situ hybridization using digoxigenin-labeled cRNA probes. Biochemica 2:21–25
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3:59–69
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116:1175–1186
Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science 287:1606–1609
Topol L et al (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162:899–908
Farr GH 3rd et al (2000) Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol 148:691–702
Nasevicius A et al (1998) Evidence for a frizzled-mediated wnt pathway required for zebrafish dorsal mesoderm formation. Development 125:4283–4292
Kane DA, Kimmel CB (1993) The zebrafish midblastula transition. Development 119:447–456
Heasman J (1997) Patterning the Xenopus blastula. Development 124:4179–4191
Nishida H (2002) Patterning the marginal zone of early ascidian embryos: localized maternal mRNA and inductive interactions. Bioessays 24:613–624
Pelegri F (2003) Maternal factors in zebrafish development. Dev Dyn 228:535–554
Wagner DS et al (2004) Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell 6:781–790
Amacher SL et al (2002) The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129:3311–3323
Gansner JM, Gitlin JD (2008) Essential role for the alpha 1 chain of type VIII collagen in zebrafish notochord formation. Dev Dyn 237:3715–3726
Gansner JM et al (2007) Essential role of lysyl oxidases in notochord development. Dev Biol 307:202–213
Hawkins TA et al (2008) The small molecule Mek1/2 inhibitor U0126 disrupts the chordamesoderm to notochord transition in zebrafish. BMC Dev Biol 8:42
Krens SF et al (2006) Characterization and expression patterns of the MAPK family in zebrafish. Gene Expr Patterns 6:1019–1026
Dietz UH et al (1999) Spatio-temporal distribution of chondromodulin-I mRNA in the chicken embryo: expression during cartilage development and formation of the heart and eye. Dev Dyn 216:233–243
Domowicz M et al (1995) The biochemically and immunologically distinct CSPG of notochord is a product of the aggrecan gene. Dev Biol 171:655–664
Sachdev SW et al (2001) Sequence analysis of zebrafish chondromodulin-1 and expression profile in the notochord and chondrogenic regions during cartilage morphogenesis. Mech Dev 105:157–162
Zhao Q et al (1997) Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn 209:377–386
Schlombs K, Wagner T, Scheel J (2003) Site-1 protease is required for cartilage development in zebrafish. Proc Natl Acad Sci USA 100:14024–14029
Holley SA, Nusslein-Volhard C (2000) Somitogenesis in zebrafish. Curr Top Dev Biol 47:247–277
Fleming A, Keynes RJ, Tannahill D (2001) The role of the notochord in vertebral column formation. J Anat 199:177–180
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 30871407), the “Chinese 111 project” (Grant no. B06018) and the “863 Program” (Grant no. 2007AA06Z407).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Xu, Y., Li, J. et al. Znrg, a novel gene expressed mainly in the developing notochord of zebrafish. Mol Biol Rep 37, 2199–2205 (2010). https://doi.org/10.1007/s11033-009-9702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9702-4