Abstract
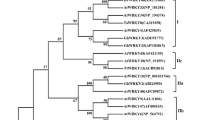
The ThPOD1 gene encodes a peroxidase and was isolated from a Tamarix hispida NaCl-stress root cDNA library. We found that ThPOD1 expression could be induced by abiotic stresses such as cold, salt, drought and exogenous abscisic acid. These findings suggested that ThPOD1 might be involved in the plant response to environmental stresses and ABA treatment. To elucidate the function of this gene, recombinant plasmids expressing full-length ThPOD1 as well as ThPOD2 (aa 41-337), and ThPOD3 (aa 73-337) truncated polypeptides were constructed. SDS–PAGE and Western blot analyses of the fusion proteins revealed that the molecular weights of ThPOD1, ThPOD2 and ThPOD3 were ~57, ~50 and ~47 kDa, respectively. Stress assays of E. coli treated with the recombinant plasmids indicated that ThPOD3 could improve resistance to drought stress. This finding could potentially be used to improve plant tolerance to drought stress via gene transfer.




Similar content being viewed by others
References
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265. doi:10.1007/s00299-005-0972-6
Bakalovic N, Passardi F, Ioannidis V, Cosio C, Penel C, Falquet L et al (2006) PeroxiBase: a class III plant peroxidase database. Phytochemistry 67:534–539. doi:10.1016/j.phytochem.2005.12.020
Morohashi Y (2002) Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radicle protrusion. J Exp Bot 53:1643–1650. doi:10.1093/jxb/erf012
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134. doi:10.1104/pp.125.1.131
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540. doi:10.1016/j.tplants.2004.09.002
Lopez-Serrano M, Fernandez MD, Pomar F, Pedreno MA, Ros Barcelo A (2004) Zinnia elegans uses the same peroxidase isoenzyme complement for cell wall lignification in both single-cell tracheary elements and xylem vessels. J Exp Bot 55:423–431. doi:10.1093/jxb/erh036
Ranieri A, Petacco F, Castagna A, Soldatini GF (2000) Redox state and peroxidase system in sunflower plants exposed to ozone. Plant Sci 159:159–167. doi:10.1016/S0168-9452(00)00352-6
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsu HI (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468. doi:10.1093/pcp/pce061
Delannoy E, Al Jallou, Assigbetse K, Marmey P, Geiger JP, Lherminier J et al (2003) Activity of class III peroxidases in the defense of cotton to bacterial blight. Mol Plant Microbe Interact 16:1030–1038. doi:10.1094/MPMI.2003.16.11.1030
Dowd PF, Johnson ET (2005) Association of a specific cationic peroxidase isozyme with maize stress and disease resistance responses, genetic identification, and identification of a cDNA coding for the isozyme. J Agric Food Chem 53:4464–4470. doi:10.1021/jf0404750
Lo’pez-Molina D, Heering HA, Smulevich G, Tudela J, Thorneley RN, Garc’ıa-Ca’novas F et al (2003) Purification and characterization of a new cationic peroxidase from fresh flowers of Cynara scolymus L. J Inorg Biochem 94:243–254. doi:10.1016/S0162-0134(02)00650-5
Marjamaa K, Hilde’n K, Kukkola E, Lehtonen M, Holkeri H, Haapaniemi P et al (2006) Cloning, characterization and localization of three novel class III peroxidases in lignifying xylem of Norway spruce (Picea abies). Plant Mol Biol 61:719–732. doi:10.1007/s11103-006-0043-6
Tognolli M, Penel C, Greppin H, Simon P (2002) Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 288:129–138. doi:10.1016/S0378-1119(02)00465-1
Hiraga S, Yamamoto K, Ito H, Sasaki K, Matsui H, Honma M et al (2000) Diverse expression profiles of 21 rice peroxidase genes. FEBS Lett 471:245–250. doi:10.1016/S0014-5793(00)01409-5
Valerio L, De Meyer M, Penel C, Dunand C (2004) Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65:1331–1342. doi:10.1016/j.phytochem.2004.04.017
Li HY, Wang YC, Jiang J, Liu GF, Gao CQ, Yang CP (2009) Identification of genes responsive to salt stress on Tamarix hispida roots. Gene 433(1–2):65–71
Liu Y, Zheng YZ (2005) PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochem Biophys Res Commun 331:325–332. doi:10.1016/j.bbrc.2005.03.165
Jaakola L, Pirttila AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19:201–213. doi:10.1385/MB:19:2:201
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Chen JM, Gao C, Shi Q, Shan B, Lei YJ, Dong CF et al (2008) Different expression patterns of CK2 subunits in the brains of experimental animals and patients with transmissible spongiform encephalopathies. Arch Virol 153:1013–1020. doi:10.1007/s00705-008-0084-z
Park SY, Ryu SH, Kwon SY, Lee HS, Kim JG, Kwak SS (2003) Differential expression of six novel peroxidase cDNAs from cell cultures of sweetpotato in response to stress. Mol Genet Genomics 269:542–552. doi:10.1007/s00438-003-0862-y
Parra-Lobato MC, Alvarez-Tinaut MC, Gomez-Jimenez MC (2007) Cloning and characterization of a root sunflower peroxidase gene putatively involved in cell elongation. J Plant Physiol 164:1688–1692. doi:10.1016/j.jplph.2007.05.006
Agrawal GK, Rakwal R, Jwa NS, Agrawal VP (2002) Characterization of a novel rice gene OsATX and modulation of its expression by components of the stress signaling pathways. Physiol Plant 116:87–95. doi:10.1034/j.1399-3054.2002.1160111.x
Shinozaki K, Yamaguchi-Shinozakiy K, Sekiz M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417. doi:10.1016/S1369-5266(03)00092-X
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG et al (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophys Res Commun 353:299–305. doi:10.1016/j.bbrc.2006.12.027
Lan Y, Cai D, Zheng YZ (2005) Expression of three different group soybean lea genes enhanced stress tolerance in Escherichia coli. Acta Bot Sin
Yamada A, Sekifuchi M, Mimura T, Ozeki Y (2002) The role of plant CCTa in salt- and osmotic-stress tolerance. Plant Cell Physiol 43:1043–1048. doi:10.1093/pcp/pcf120
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279. doi:10.1146/annurev.arplant.49.1.249
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant 100:241–254. doi:10.1111/j.1399-3054.1997.tb04780.x
Schweizer P (2008) Tissue-specific expression of a defence-related peroxidase in transgenic wheat potentiates cell death in pathogen-attacked leaf epidermis. Mol Plant Pathol 9:45–57
Kim YH, Kim CY, Song WK, Park DS, Kwon SY, Lee HS et al (2008) Overexpression of sweetpotato swpa4 peroxidase results in increased hydrogen peroxide production and enhances stress tolerance in tobacco. Planta 227:867–881. doi:10.1007/s00425-007-0663-3
Mittler R, Zilinskas BA (1992) Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem 267:21802–21807
Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG (1997) Responses of antioxidants to paraquat in pea leaves. Plant Physiol 113:249–257
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657. doi:10.1126/science.284.5414.654
Acknowledgments
This study was supported by national natural science foundation (Grant No. 30571509), Heilongjiang province scientific and technological project (Grant No. GB06B303 and WB07N02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, XH., Jiang, J., Wang, BC. et al. ThPOD3, a truncated polypeptide from Tamarix hispida, conferred drought tolerance in Escherichia coli . Mol Biol Rep 37, 1183–1190 (2010). https://doi.org/10.1007/s11033-009-9484-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9484-8