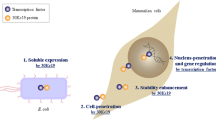
Abstract
HSF1 is the major transcription factor of HSPs (heat shock proteins) in response to various stresses. Wild type HSF1 (heat shock transcriptional factor 1) is normally inactive, while a constitutively active form of HSF1 (HSF1(+)) can activate downstream HSP expression in the absence of stresses. Here we generated the eukaryotic vectors that expresses HSF1(+) fusion proteins, and found that HSF1(+)-TAT fusion protein was expressed and activated HSP expression. TAT, as a trans-acting factor of HIV-1, has been demonstrated to deliver functional cargo protein into living cells. HSF1(+)-TAT fusion protein was expressed in E. coli, purified, incubated with A549 cells for 8 h, Western blot analysis and luciferase reporter assay showed that HSF1(+) fusion protein was delivered into A549 cells successfully, and the accumulation of HSF1(+)-TAT fusion protein in A549 cells up-regulated HSP70 expression.





Similar content being viewed by others
References
Voellmy R (2004) On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones 9:122–133. doi:10.1379/CSC-14R.1
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11:441–469. doi:10.1146/annurev.cb.11.110195.002301
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796. doi:10.1101/gad.12.24.3788
Kingston RE, Schuetz TJ, Larin Z (1987) Heat-inducible human factor that binds to a human HSP70 promoter. Mol Cell Biol 7:1530–1534
Wu C (1984) Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature 311:81–84. doi:10.1038/311081a0
Zimarino V, Tsai C, Wu C (1990) Complex modes of heat shock factor activation. Mol Cell Biol 10:752–759
Sarge KD, Murphy SP, Morimoto RI (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol 13:1392–1407
Baler R, Dahl G, Voellmy R (1993) Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol 13:2486–2496
Jones TJ, Li D, Wolf IM et al (2004) Enhancement of glucocorticoid receptor-mediated gene expression by constitutively active heat shock factor 1. Mol Endocrinol 18:509–520. doi:10.1210/me.2003-0366
Zuo J, Rungger D, Voellmy R (1995) Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol 15:4319–4330
Voellmy R (2005) Dominant-positive and dominant-negative heat shock factors. Methods 35:199–207. doi:10.1016/j.ymeth.2004.08.010
Green M, Loewenstein PM (1988) Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179–1188. doi:10.1016/0092-8674(88)90262-0
Frankel AD, Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193. doi:10.1016/0092-8674(88)90263-2
Fawell S, Seery J, Daikh Y et al (1994) Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA 91:664–668. doi:10.1073/pnas.91.2.664
Schwarze SR, Ho A, Vocero-Akbani A et al (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569–1572. doi:10.1126/science.285.5433.1569
Becker-Hapak M, McAllister SS, Dowdy SF (2001) TAT-mediated protein transduction into mammalian cells. Methods 24:247–256. doi:10.1006/meth.2001.1186
Rabindran SK, Giorgi G, Clos J et al (1991) Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci USA 88:6906–6910. doi:10.1073/pnas.88.16.6906
Pardhasaradhi K, Kutty RK, Park CS et al (1994) Cloning and sequencing of heat shock factor (HSF1) cDNA from human retina. Curr Eye Res 13:739–742. doi:10.3109/02713689409047008
Soncin F, Prevelige R, Calderwood SK (1997) Expression and purification of human heat-shock transcription factor 1. Protein Expr Purif 9:27–32. doi:10.1006/prep.1996.0672
Ryu J, Han K, Park J et al (2003) Enhanced uptake of a heterologous protein with an HIV-1 Tat protein transduction domains (PTD) at both termini. Mol Cells 16:385–391
Drees BL, Grotkopp EK, Nelson HC (1997) The GCN4 leucine zipper can functionally substitute for the heat shock transcription factor’s trimerization domain. J Mol Biol 273:61–74. doi:10.1006/jmbi.1997.1283
Harrison CJ, Bohm AA, Nelson HC (1994) Crystal structure of the DNA binding domain of the heat shock transcription factor. Science 263:224–227. doi:10.1126/science.8284672
Damberger FF, Pelton JG, Harrison CJ et al (1994) Solution structure of the DNA-binding domain of the heat shock transcription factor determined by multidimensional heteronuclear magnetic resonance spectroscopy. Protein Sci 3:1806–1821
Sorger PK, Nelson HC (1989) Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell 59:807–813. doi:10.1016/0092-8674(89)90604-1
Peteranderl R, Nelson HC (1992) Trimerization of the heat shock transcription factor by a triple-stranded alpha-helical coiled-coil. Biochemistry 31:12272–12276. doi:10.1021/bi00163a042
Ishihara K, Horiguchi K, Yamagishi N et al (2003) Identification of sodium salicylate as an HSP inducer using a simple screening system for stress response modulators in mammalian cells. Eur J Biochem 270:3461–3468. doi:10.1046/j.1432-1033.2003.03740.x
Zou Y, Zhu W, Sakamoto M et al (2003) Heat shock transcription factor 1 protects cardiomyocytes from ischemia/reperfusion injury. Circulation 108:3024–3030. doi:10.1161/01.CIR.0000101923.54751.77
Batulan Z, Taylor DM, Aarons RJ et al (2006) Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis 24:213–225. doi:10.1016/j.nbd.2006.06.017
Bruening W, Roy J, Giasson B et al (1999) Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J Neurochem 72:693–699. doi:10.1046/j.1471-4159.1999.0720693.x
Nagahara H, Vocero-Akbani AM, Snyder EL et al (1998) Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4:1449–1452. doi:10.1038/4042
Derossi D, Joliot AH, Chassaing G, Prochiantz A (1994) The third helix of the antennapedia homeodomain translocates through biological membranes. J Biol Chem 269:10444–10450
Schwarze SR, Hruska KA, Dowdy SF (2000) Protein transduction: unrestricted delivery into all cells? Trends Cell Biol 10:290–295. doi:10.1016/S0962-8924(00)01771-2
Elliott G, O’Hare P (1997) Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88:223–233. doi:10.1016/S0092-8674(00)81843-7
Wheeler DS, Dunsmore KE, Wong HR (2003) Intracellular delivery of HSP70 using HIV-1 TAT protein transduction domain. Biochem Biophys Res Commun 301:54–59. doi:10.1016/S0006-291X(02)02986-8
Lai Y, Du L, Dunsmore KE, Jenkins LW et al (2005) Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J Neurochem 94:360–366. doi:10.1111/j.1471-4159.2005.03212.x
Acknowledgments
The research was supported by the grants from Guangdong Natural Science Foundation (6026372) and Guangdong Institute of Chinese Medicine (1060165). We thank Dr. Richard Voellmy for the kind gift of pcDNA-HSF1(+) plasmid.