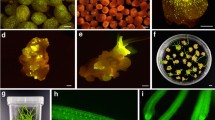
Abstract
The Nicotiana tabacum × N. africana cross is a semilethal cross, where greater than 99 % of progeny die in the cotyledonary stage due to an interspecific lethality reaction. The low frequencies of surviving plants are mixtures of maternal tobacco haploids and aneuploid F1 hybrids. For effective use of surviving haploids in a breeding program, an efficient method is needed to distinguish them from aneuploid hybrids during the seedling stage. The first objective of this research was to investigate the use of N. africana engineered with a green fluorescent protein (gfp) transgene for distinguishing haploids from other surviving plants derived from this cross. Results demonstrated gfp expression to be a useful phenotypic marker for separation of aneuploid hybrids at the seedling stage. Microsatellite marker genotyping, flow cytometry determinations, and chromosome counts resulted in identification of progeny that exhibited intermediate DNA amounts and variable chromosome numbers donated by the N. africana parent. Three individuals with near-haploid DNA content were identified that carried N. africana markers and n = 24 + 2 or n = 24 + 3 chromosomes. The results present evidence of genetic instability in progeny from the N. tabacum × N. africana cross and suggest that, in contrast to previous belief, chromosome elimination may play at least a partial role in the generation of haploids from this cross. Additionally, microsatellite marker genotyping demonstrated a role for a gene or genes located near the end of N. tabacum chromosome H (linkage group 11) in the interspecific lethality reaction.




Similar content being viewed by others
References
Adachi T (2001) Introduction and concept of breeding barriers. In: Adachi T, Imanishi S, Hoffmann F (eds) How to overcome breeding barriers by means of plant biotechnology: a case study in tomato. Osaka Municipal Universities Press, Osaka, pp 1–4
An G, Watson BD, Cheng CC (1986) Transformation of tobacco, tomato, potato, and Arabidopsis thaliana using a binary Ti vector system. Plant Physiol 891:301–305
Andres RJ, Kuraparthy V (2013) Development of an improved method of mitotic metaphase chromosome preparation compatible for fluorescence in situ hybridization in cotton. J Cotton Sci 17:149–156
Bennett MD, Finch RA, Barclay IR (1976) The time rate and mechanism of chromosome elimination in Hordeum hybrids. Chromosoma 54:175–200
Bindler G, Pileske J, Bakaher N, Gunduz I, Ivanov N, van der Hoeven R, Ganal M, Donini P (2011) A high density genetic map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development. Theor Appl Genet 23:219–230
Bourgin J-P, Nitsch JP (1967) Production of haploid Nicotiana from excised stamens. Ann Physiol Veg 9:377–382
Burk LG (1962) Haploids in genetically marked progenies of tobacco. J Hered 53:222–225
Burk LG, Gerstel DU, Wernsman EA (1979) Maternal haploids of Nicotiana tabacum L. from seed. Science 206:585
Burns JA (1964) A technique for making preparations of mitotic chromosomes from Nicotiana flowers. Tob Sci 8:1–2
Chen CX, Zhu LH, Sun JS (1998) Molecular evidence on maize specific DNA fragment transferred into wheat through sexual hybridization. Sci China Ser C-Life Sci 41:126–132
Chiu WL, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Clausen RE, Cameron DR (1944) Inheritance in Nicotiana tabacum. XVIII. Monosomic analysis. Genetics 29:447–477
Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY (1995) Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20:448–455
Davies DR (1974) Chromosome elimination in inter-specific hybrids. J Hered 32:267–270
de Nettancourt D, Stokes GW (1960) Haploidy in tobacco. J Hered 51:102–104
Finch RA (1983) Tissue-specific elimination of alternative whole parental genomes in one barley hybrid. Chromosoma 88:386–393
Flowers RA, Stokes GW, Smiley JH (1967) Identification of tobacco haploids by stomatal size. Tob Sci 11:72–74
Gernand D, Rutten T, Varshney A, Rubtsova M, Prodanovic S, Brüß C, Kumlehn J, Matzk F, Houben A (2005) Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell 17:2431–2438
Gernand D, Rutten T, Pickering R, Houben A (2006) Elimination of chromosomes in Hordeum vulgare × H. bulbosum crosses at mitosis and interphase involves micronucleus formation and progressive heterochromatization. Cytogenet Genome Res 114:169–174
Gerstel DU (1945) Inheritance in Nicotiana tabacum. XIX. Identification of the tabacum chromosome replaced by one from N. glutinosa in mosaic-resistant Holmes Samsoun tobacco. Genetics 30:448–454
Gerstel DU, Burk LG (1960) Controlled introgression in Nicotiana: a cytological study. Tob Sci 4:147–150
Gerstel DU, Burns JA, Burk LG (1979) Interspecific hybridizations with an African tobacco, Nicotiana africana Merxm. J Hered 70:342–344
Gupta SB (1969) Duration of mitotic cycle and regulation of DNA replication in Nicotiana plumbaginifolia and a hybrid derivative of N. tabacum showing chromosome instability. Can J Genet Cytol 11:133–142
Haseloff J, Siemering KR, Prasher D, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Heim R, Prasher DC, Tsien RY (1994) Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA 91:12501–12504
Ishii T, Ueda T, Tanaka H, Tsujimoto H (2010) Chromosome elimination by wide hybridization between Triticeae or oat plant and pearl millet: pearl millet chromosome dynamics in hybrid embryo cells. Chromosome Res 18:821–831
Kasha KJ, Kao KN (1970) High frequency haploid production in barley (Hordeum vulgare L.). Nature 225:874–876
Kim NS, Armstrong KC, Fedak G, Ho K, Park NI (2002) A microsatellite sequence from the rice blast fungus (Magnaporthe grisea) distinguishes between the centromeres of Hordeum vulgare and H. bulbosum in hybrid plants. Genome 45:165–174
Kramer MG, Reed SM (1988) An evaluation of maternal nullihaploidy for Nicotiana tabacum L. nullisomic production. I. An interspecific hybridization approach. J Hered 79:24–27
Kynast RG, Riera-Lizarazu O, Vales MI, Okagaki RJ, Maquieira SB, Chen G, Ananiev EV, Odland WE, Russell CD, Stec AO, Livingston SM, Zaia HA, Rines HW, Philips RL (2001) A complete set of maize individual chromosome additions to the oat genome. Plant Physiol 125:1216–1227
Laurie DA, Bennett MD (1989) The timing of chromosome elimination in hexaploid wheat × maize crosses. Genome 32:953–961
Lewis RS (2003) Unpublished information. North Carolina State University
Lewis RS, Rose C (2011) Identification of tobacco haploids on the basis of transgenic overexpression of PAP1 from Arabidopsis thaliana. Crop Sci 51:1491–1497
RS, Milla SR, Levin JS (2005) Molecular and genetic characterization of Nicotiana glutinosa L. chromosome segments in tobacco mosaic virus-resistant tobacco accessions. Crop Sci 45:2355–2362
Li L, Xu X, Jin W, Chen S (2009) Morphological and molecular evidences for DNA introgression in haploid induction via a high oil inducer CAUHOI in maize. Planta 230:367–376
Linde-Laursen I, von Bothmer R (1999) Orderly arrangement of the chromosomes within barley genomes of chromosome-eliminating Hordeum lechleri × barley hybrids. Genome 42:225–236
Lucas GB, Gooding GV Jr, Sasser JN, Gerstel DU (1980) Reaction of Nicotiana africana to black shank, Granville wilt, root knot, tobacco mosaic virus, and potato virus Y. Tob Sci 24:141–142
Michel B (2000) Replication fork arrest and DNA recombination. Trends Biochem Sci 25:173–178
Mino M, Maekawa K, Ogawa K, Yamagishi H, Inoue M (2002) Cell death processes during expression of hybrid lethality in interspecific F1 hybrid between Nicotiana gossei Domin and Nicotiana tabacum. Plant Physiol 130:1776–1787
Mochida K, Tsujimoto H, Sasakuma T (2004) Confocal analysis of chromosome behavior in wheat × maize zygotes. Genome 47:199–205
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Pelletier G, Ferault M, Goujaud J, Vedel F, Caboche M (1987) The use of rootless mutants for the screening of spontaneous androgenetic and gynogenetic haploids in Nicotiana tabacum: evidence for the direct transfer of cytoplasm. Theor Appl Genet 75:13–15
Ravi M, Chan SWL (2010) Haploid plants produced by centromere-mediated genome elimination. Nature 464:615–618
Reed SM (1993) Use of stomatal size to distinguish between haploid and dihaploid tobacco plants. Tob Sci 37:84–86
Riera Lizarazu O, Rines HW, Phillips RL (1996) Cytological and molecular characterization of oat × maize partial hybrids. Theor Appl Genet 93:123–135
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanei M, Pickering R, Kumke K, Nasuda S, Houben A (2011) Loss of centromeric histone H3 (CENH3) from centromeres precedes uniparental chromosome elimination in interspecific barley hybrids. Proc Natl Acad Sci USA 108:498–505
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Schwarzacher Robinson T, Finch RA, Smith JB, Bennett MD (1987) Genotypic control of centromere positions of parental genomes in Hordeum × Secale hybrid metaphases. J Cell Sci 87:291–304
Siemering KR, Golbik R, Sever R, Haseloff J (1996) Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol 6:1653–1663
Stines BJ, Mann TG (1960) Diploidization in Nicotiana tabacum: a study of the yellow burley character. J Hered 51:222–227
Subrahmanyam NC, Kasha KJ (1973) Selective chromosomal elimination during haploid formation in barley following interspecific hybridization. Chromosoma 42:111–125
Tezuka T (2012) Hybrid lethality in the genus Nicotiana. In: Mworia JK (ed) Botany. InTech, Rijeka, pp 191–210
Tezuka T, Matsuo C, Lizuka T, Oda M, Marubashi W (2012) Identification of Nicotiana tabacum linkage group corresponding to the Q chromosome gene(s) involved in hybrid lethality. PLoS One 7:e37822
Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67:509–544
Wernsman EA (1992) Varied roles for the haploid sporophyte in plant improvement. In: Stalker HT, Murphy JP (eds) Plant breeding in the 1990s. CAB Int, Wallingforde, pp 461–481
Wernsman EA, Matzinger DF, Rufty RC (1989) Androgenetic vs. gynogenetic doubled haploids of tobacco. Crop Sci 29:1151–1155
Wilkinson MJ, Bennett ST, Clulow SA, Allainguillaume J, Harding K, Bennett MD (1995) Evidence for somatic translocation during potato dihaploid induction. Heredity 74:146–151
Wu F, Eanetta NT, Xu Y, Plieske J, Ganal M, Pozzi C, Bakaher N, Tanksley SD (2009) COSII genetics maps of two diploid Nicotiana species provide a detailed picture of synteny with tomato and insights into chromosome evolution in tetraploid N. tabacum. Theor Appl Genet 120:809–827
Zhang ZL, Qiu FZ, Liu YZ, Ma KJ, Li ZY, Zu SZ (2008) Chromosome elimination and in vivo haploid production induced by Stock 6-derived inducer line of maize (Zea mays L.). Plant Cell Rep 27:1851–1860
Zhao Z, Xu X, Xie H, Chen S, Jin W (2013) Fertilization and uniparental chromosome elimination during crosses with maize haploid inducers. Plant Physiol 163:721–731
Acknowledgments
We thank the laboratory of Dr. George Allen at N.C. State University for providing us with the 35S:mgfp5-ER construct. We also gratefully acknowledge the financial support of our research program by Philip Morris International and Altria Client Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
WGH performed the research, helped to analyze the data, and helped draft the manuscript; VK conducted cytological analyses; SPK performed plant transformations; RSL designed the research, helped analyze the data, and wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hancock, W.G., Kuraparthy, V., Kernodle, S.P. et al. Identification of maternal haploids of Nicotiana tabacum aided by transgenic expression of green fluorescent protein: evidence for chromosome elimination in the N. tabacum × N. africana interspecific cross. Mol Breeding 35, 179 (2015). https://doi.org/10.1007/s11032-015-0372-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0372-8