Abstract
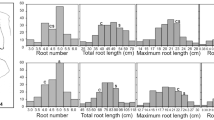
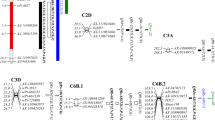
Roots are essential for normal growth, development, and reproduction of higher plants. Consequently, improvement of root system architecture and functionality is of fundamental importance in crop improvement. However, the genetic mechanisms controlling root morphology and function are still not well understood, especially in common wheat, which possesses a complex and unsequenced hexaploid genome. Here we report a more detailed genetic analysis of qTaLRO-B1, a major quantitative trait locus (QTL) previously detected to affect root length and related traits in common wheat. A pair of QTL isolines with different qTaLRO-B1 alleles was developed. Line 178B, carrying the longer root allele, was significantly more efficient in taking up phosphate nutrient and biomass accumulation than line 178A, with the shorter root allele. We mapped qTaLRO-B1 to a 0.9-cM interval on common wheat chromosome 2BS with seven sequence-tagged-site (STS) markers developed from the genes conserved between wheat and Brachypodium distachyon. The seven STS markers were collinearly conserved in tetraploid wheat, but they covered a much larger genetic distance (22.8 cM) in the latter species. In conclusion, we have converted qTaLRO-B1 into a major gene that affects common wheat root length in a qualitative manner, and improved understanding of the genetic location of qTaLRO-B1 and the chromosomal segment carrying this important locus. The implications of our data for further study of qTaLRO-B1 are discussed.




Similar content being viewed by others
References
Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF (2006) A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325
An DG, Su JY, Liu QY, Li B, Jing RL, Li JY, Li ZS (2006) Mapping QTLs for nitrogen uptake in relation to the early growth of wheat (Triticum aestivum L.). Plant Soil 284:73–84
Bai C, Liang Y, Hawkesford MJ (2013) Identification of QTLs associated with seedling root traits and their correlation with plant height in wheat. J Exp Bot 64:1745–1753
Chernoff H, Lehmann EL (1954) The use of maximum likelihood estimates in χ2 tests for goodness-of-fit. Ann Math Stat 25:579–586
Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi AM, Jordan D, Mace E, Hammer G (2013) QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor Appl Genet 126:1563–1574
Coudert Y, Périn C, Courtois B, Khong NG, Gantet P (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci 15:219–226
Creste S, Neto T, Figueira A (2001) Detection of single sequence repeat polymorphisms in denaturing polyacrylamide sequencing gels by silver staining. Plant Mol Biol Rep 19:299–306
Den Herder G, Van Isterdael G, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 15:600–607
Dixon JM (2009) Wheat facts and futures 2009. CIMMYT, Mexico
Dunbabin V, Diggle A, Rengel Z (2003) Is there an optimal root architecture for nitrate capture in leaching environments? Plant Cell Environ 26:835–844
Ehdaie B, Merhaut DJ, Ahmadian S, Hoops AC, Khuong T, Layne AP, Waines JG (2010) Root system size influences water-nutrient uptake and nitrate leaching potential in wheat. J Agron Crop Sci 196:455–466
Fita A, Picó B, Monforte AJ, Nuez F (2008) Genetics of root system architecture using near-isogenic lines of melon. J Am Soc Hort Sci 133:448–458
Gill KS, Gill BS, Endo TR, Boyko EV (1996a) Identification and high-density mapping of gene rich regions in chromosome group 5 of wheat. Genetics 143:1001–1012
Gill KS, Gill BS, Endo TR, Taylor T (1996b) Identification and high-density mapping of gene rich regions in chromosome group 1 of wheat. Genetics 144:1883–1891
Good A, Beatty P (2011) Biotechnological approaches to improving nitrogen use efficiency in plants: alanine aminotransferase as a case study. In: Hawkesford MJ, Barraclough P (eds) The molecular and physiological basis of nutrient use efficiency in crops. Wiley-Blackwell, Oxford. doi:10.1002/9780470960707.ch9
Hamada A, Nitta M, Nasuda S, Kato K, Fujita M, Matsunaka H, Okumoto Y (2012) Novel QTLs for growth angle of seminal roots in wheat (Triticum aestivum L.). Plant Soil 354:395–405
Jia Y (2009) Artificial introgression of a large fragment around the Pi-ta rice blast resistance gene in backcross progenies and several elite rice cultivars. Heredity 103:333–339
Jia Y, Jia MH, Wang Z, Liu G (2012) Indica and Japonica crosses resulting in linkage block and recombination suppression on rice chromosome 12. PLoS ONE 7:e43066
Jung JK, McCouch S (2013) Getting to the roots of it: genetic and hormonal control of root architecture. Front Plant Sci 4:186
Kim JS, Islam-Faridi MN, Klein PE, Stelly DM, Price HJ, Klein RR, Mullet JE (2005) Comprehensive molecular cytogenetic analysis of sorghum genome architecture: distribution of euchromatin, heterochromatin, genes and recombination in comparison to rice. Genetics 171:1963–1976
Landi P, Sanguineti MC, Liu C, Li Y, Wang TY, Giuliani S, Bellotti M, Salvi S, Tuberosa R (2007) Root-ABA1 QTL affects root lodging, grain yield and other agronomic traits in maize grown under well-watered and water-stressed conditions. J Exp Bot 58:319–326
Landi P, Giuliani S, Salvi S, Ferri M, Tuberosa R, Sanguineti MC (2010) Characterization of root-yield-1.06, a major constitutive QTL for root and agronomic traits in maize across water regimes. J Exp Bot 61:3553–3562
Landjeva S, Neumann K, Lohwasser U, Börner A (2008) Molecular mapping of genomic regions associated with wheat seedling growth under osmotic stress. Biol Plant 52:259–266
Liang Q, Cheng X, Mei M, Yan X, Liao H (2010) QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann Bot 106:223–234
Liu Z, Zhu J, Cui Y, Liang Y, Wu H, Song W, Liu Q, Yang T, Sun Q, Liu Z (2012) Identification and comparative mapping of a powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides) on chromosome 2BS. Theor Appl Genet 124:1041–1049
Liu X, Li R, Chang X, Jing R (2013) Mapping QTLs for seedling root traits in a doubled haploid wheat population under different water regimes. Euphytica 189:51–66
MacKey J (1979) Genetic potentials for improved yield. Acta Agron Hung 28:121–143
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell Environ 28:67–77
Manschadi AM, Hammer GL, Christopher JT, de Voil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Mohler V, Lukman R, Ortiz-Islas S, William M, Worland AJ, van Beem J, Wenzel G (2004) Genetic and physical mapping of photoperiod insensitive gene Ppd-B1 in common wheat. Euphytica 138:33–40
Murphy JR, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Neu C, Stein N, Keller B (2002) Genetic mapping of the Lr20-Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome 45:737–744
Obara M, Tamura W, Ebitani T, Yano M, Sato T, Yamaya T (2010) Fine-mapping of qRL6.1, a major QTL for root length of rice seedlings grown under a wide range of NH4+ concentrations in hydroponic conditions. Theor Appl Genet 121:535–547
Ren Y, He X, Liu D, Li J, Zhao X, Li B, Tong Y, Zhang A, Li Z (2012) Major quantitative trait loci for seminal root morphology of wheat seedlings. Mol Breed 30:139–148
Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G (2012) Achieving yield gains in wheat. Plant Cell Environ 35:1799–1823
Ron M, Dorrity MW, de Lucas M, Toal T, Hernandez RI, Little SA, Maloof JN, Kliebenstein DJ, Brady SM (2013) Identification of novel loci regulating interspecific variation in root morphology and cellular development in tomato. Plant Physiol 162:755–768
Rosegrant MW, Agcaoili M (2010) Global food demand, supply, and price prospects to 2010. International Food Policy Research Institute, Washington
Rosegrant MW, Ringler C, Zhu T (2009) Water for agriculture: maintaining food security under growing scarcity. Annu Rev Environ Res 34:205–222
Sanguineti MC, Li S, Maccaferri M, Corneti S, Rotondo F, Chiari T, Tuberosa R (2007) Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann Appl Biol 151:291–305
Sears ER (1954) The aneuploids of common wheat. MO Agr Exp Sta Res Bull 572. University of Missouri, Columbia
Sharma S, Xu S, Ehdaie B, Hoops A, Clsoe TJ, Lukaszewski AJ, Waines JG (2011) Dissection of QTL effects for root traits using a chromosome arm-specific mapping population in bread wheat. Theor Appl Genet 122:759–769
Shen B, Courtois B, McNally KL, Robin S, Li Z (2001) Evaluation of near-isogenic lines of rice introgressed with QTLs for root depth through marker-aided selection. Theor Appl Genet 103:75–83
Su JY, Zheng Q, Li HW, Li B, Jing RL, Tong YP, Li ZS (2009) Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Sci 176:824–836
Suji KK, Prince K, Mankhar PS, Kanagaraj P, Poornima R, Amutha K, Kavitha S, Biji KR, Gomez SM, Babu RC (2012) Evaluation of rice (Oryza sativa L.) near iso-genic lines with root QTLs for plant production and root traits in rainfed target populations of environment. Field Crop Res 137:89–96
Topp CN, Iyer-Pascuzzi AS, Anderson JT, Lee CR, Zurek PR, Symonova O, Zheng Y, Bucksch A, Mileyko Y, Galkovskyi T, Moore BT, Harer J, Edelsbrunner H, Mitchell-Olds T, Weitz JS, Benfey PN (2013) 3D phenotyping and quantitative trait locus mapping identify core regions of the rice genome controlling root architecture. Proc Natl Acad Sci USA 110:1695–1704
Tuberosa R, Salvi S (2007) From QTLs to genes controlling root traits in maize. In: Spiertz JHJ, Struik PC, van Laar HH (eds) Scale and complexity in plant systems research: gene–plant–crop relations. Springer, The Netherlands, pp 13–22
Tuberosa R, Salvi S, Sanguineti MC, Landi P, Maccaferri M, Conti S (2002a) Mapping QTLs regulating morpho-physiological traits and yield: case studies, shortcomings and perspectives in drought-stressed maize. Ann Bot 89:941–963
Tuberosa R, Sanguineti MC, Landi P, Michela Giuliani M, Salvi S (2002b) Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol Biol 48:697–712
Tuinstra MR, Ejeta G, Goldsbrough PB (1997) Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95:1005–1011
Van Ooijen JW, Voorrips RE (2006) JoinMap4.0, software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Ann Bot 100:991–998
Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 6:3485–3498
White PJ, George TS, Dupuy LX, Karley AJ, Valentine TA, Wiesel L, Wishart J (2013) Root traits for infertile soils. Front Plant Sci 4:193
Yan HL, Liu WK, Li GH, Zhang SX (2010) Comparison of rhizosphere impacts of wheat genotypes differing in phosphorus efficiency. Can J Plant Sci 90:311–317
Yonemaru JI, Yamamoto T, Ebana K, Yamamoto E, Nagasaki H, Shibaya T, Yano M (2012) Genome-wide haplotype changes produced by artificial selection during modern rice breeding in Japan. PLoS ONE 7:e32982
You FM, Huo N, Gu YQ, Lazo GR, Dvorak J, Anderson OD (2009) Conserved Primers 2.0: a high-throughput pipeline for comparative genome referenced intron-flanking PCR primer design and its application in wheat SNP discovery. BMC Bioinform 10:331
Zhang J, Zheng HG, Aarti A, Pantuwan G, Nguyen TT, Tripathy JN, Sarial AK, Robin S, Babu RC, Nguyen BD et al (2001) Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species. Theor Appl Genet 103:19–29
Zhang J, Ju XT, Gao Q, Zhang FS (2005) Recovery of labeled nitrate-N in different soil layers by two crops, spinach and wheat. Sci Agric Sin 38:333–340
Zhang K, Wang J, Zhang L, Rong C, Zhao F, Peng T, Li H, Cheng D, Liu X, Qin H, Zhang A, Tong Y, Wang D (2013) Association analysis of genomic loci important for grain weight control in elite common wheat varieties cultivated with variable water and fertiliser supply. PLoS ONE 8:e57853
Acknowledgments
This research was supported by the Ministry of Science and Technology of China (via grants 2011BAD07B02-2 and 2011CB100300). We thank Professors Junhua Peng and Eviatar Nevo for supplying the wild emmer wheat accession WEW270, and Shuangjuan Yang for technical advice on data processing during linkage analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Pei Cao and Yongzhe Ren have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, P., Ren, Y., Zhang, K. et al. Further genetic analysis of a major quantitative trait locus controlling root length and related traits in common wheat. Mol Breeding 33, 975–985 (2014). https://doi.org/10.1007/s11032-013-0013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-0013-z