Abstract
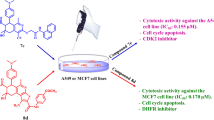
The avidity of cancer cells for iron highlights the potential for iron chelators to be used in cancer therapy. Herein, we designed and synthesized a novel series of 5H-[1,2,4]triazino[5,6-b]indole derivatives bearing a pyridinocycloalkyl moiety using a ring-fusion strategy based on the structure of an iron chelator, VLX600. The antiproliferative activity evaluation against cancer cells and normal cells led to the identification of compound 3k, which displayed the strongest antiproliferative activity in vitro against A549, MCF-7, Hela and HepG-2 with IC50 values of 0.59, 0.86, 1.31 and 0.92 μM, respectively, and had lower cytotoxicity against HEK293 than VLX600. Further investigations revealed that unlike VLX600, compound 3k selectively bound to ferrous ions, but not to ferric ions, and addition of Fe2+ abolished the cytotoxicity of 3k. Flow cytometry assays demonstrated that 3k arrested the cell cycle at the G1 phase and induced significant apoptosis in A549 cells in dose and time-dependent manners, corresponding to JC-1 staining assay results. Western blot analysis of Bcl-2, Bax and cleaved caspase-3 proteins further provided evidences that induction of apoptosis by 3k in A549 cells might be at least via the mitochondria pathway. These above results highlight that 3k is a valuable lead compound that deserves further investigation as an iron chelator for the treatment of cancer.
Graphical abstract











Similar content being viewed by others
References
Guo Q, Li L, Hou S, Yuan Z, Li C, Zhang W et al (2021) The role of iron in cancer progression. Front Oncol 11:778492. https://doi.org/10.3389/fonc.2021.778492
Torti SV, Torti FM (2020) Iron: the cancer connection. Mol Aspects Med 75:100860. https://doi.org/10.1016/j.mam.2020.100860
Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM (2018) Iron and cancer. Annu Rev Nutr 38(1):97–125. https://doi.org/10.1146/annurev-nutr-082117-051732
Abbasi U, Abbina S, Gill A, Takuechi LE, Kizhakkedathu JN (2021) Role of iron in the molecular pathogenesis of diseases and therapeutic opportunities. ACS Chem Biol 16(6):945–972. https://doi.org/10.1021/acschembio.1c00122
Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J et al (2022) Ferroptosis in cancer and cancer immunotherapy. Cancer Commun 42(2):88–116. https://doi.org/10.1002/cac2.12250
Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M (2018) Iron in the tumor microenvironment—connecting the dots. Front Oncol 8:549. https://doi.org/10.3389/fonc.2018.00549
Ibrahim O, O’Sullivan J (2020) Iron chelators in cancer therapy. Biometals 33(4–5):201–215. https://doi.org/10.1007/s10534-020-00243-3
Chen X, Kang R, Kroemer G, Tang D (2021) Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18(5):280–296. https://doi.org/10.1038/s41571-020-00462-0
Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C et al (2020) Metallodrugs are unique: opportunities and challenges of discovery and development. Chem Sci 11(48):12888–12917. https://doi.org/10.1039/d0sc04082g
Kim JL, Lee DH, Na YJ, Kim BR, Jeong YA, Lee SI et al (2016) Iron chelator-induced apoptosis via the ER stress pathway in gastric cancer cells. Tumour Biol 37(7):9709–9719. https://doi.org/10.1007/s13277-016-4878-4
Harima H, Kaino S, Takami T, Shinoda S, Matsumoto T, Fujisawa K et al (2016) Deferasirox, a novel oral iron chelator, shows antiproliferative activity against pancreatic cancer in vitro and in vivo. BMC Cancer 16(1):702. https://doi.org/10.1186/s12885-016-2744-9
Xue Y, Zhang G, Zhou S, Wang S, Lv H, Zhou L et al (2021) Iron chelator induces apoptosis in osteosarcoma cells by disrupting intracellular iron homeostasis and activating the MAPK pathway. Int J Mol Sci 22(13):7168. https://doi.org/10.3390/ijms22137168
Lan L, Wei W, Zheng Y, Niu L, Chen X, Huang D et al (2018) Deferoxamine suppresses esophageal squamous cell carcinoma cell growth via ERK1/2 mediated mitochondrial dysfunction. Cancer Lett 432:132–143. https://doi.org/10.1016/j.canlet.2018.06.012
Noulsri E, Richardson DR, Lerdwana S, Fucharoen S, Yamagishi T, Kalinowski DS et al (2009) Antitumor activity and mechanism of action of the iron chelator, Dp44mT, against leukemic cells. Am J Hematol 84(3):170–176. https://doi.org/10.1002/ajh.21350
Krishan S, Sahni S, Leck LYW, Jansson PJ, Richardson DR (2020) Regulation of autophagy and apoptosis by Dp44mT-mediated activation of AMPK in pancreatic cancer cells. Biochim Biophys Acta Mol Basis Dis 1866(5):165657. https://doi.org/10.1016/j.bbadis.2019.165657
Yuan J, Lovejoy DB, Richardson DR (2004) Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood 104(5):1450–1458. https://doi.org/10.1182/blood-2004-03-0868
Chen G, Niu C, Yi J, Sun L, Cao H, Fang Y et al (2019) Novel triapine derivative induces copper-dependent cell death in hematopoietic cancers. J Med Chem 62(6):3107–3121. https://doi.org/10.1021/acs.jmedchem.8b01996
Zhou J, Zhang L, Wang M, Zhou L, Feng X, Yu L et al (2019) CPX targeting DJ-1 triggers ROS-induced cell death and protective autophagy in colorectal cancer. Theranostics 9(19):5577–5594. https://doi.org/10.7150/thno.34663
Huang Z, Huang S (2021) Reposition of the fungicide ciclopirox for cancer treatment. Recent Pat Anti-Cancer Drug Discov 16(2):122–135. https://doi.org/10.2174/1574892816666210211090845
Fiorillo M, Toth F, Brindisi M, Sotgia F, Lisanti MP (2020) Deferiprone (DFP) targets cancer stem cell (CSC) propagation by inhibiting mitochondrial metabolism and inducing ROS production. Cells 9(6):1529. https://doi.org/10.3390/cells9061529
Simoes RV, Veeraperumal S, Serganova IS, Kruchevsky N, Varshavsky J, Blasberg RG et al (2017) Inhibition of prostate cancer proliferation by Deferiprone. NMR Biomed 30(6):10.1002/nbm.3712. https://doi.org/10.1002/nbm.3712
Yasumoto E, Nakano K, Nakayachi T, Morshed SR, Hashimoto K, Kikuchi H et al (2004) Cytotoxic activity of deferiprone, maltol and related hydroxyketones against human tumor cell lines. Anticancer Res 24(2b):755–762
Jakobsson AW, Kundu S, Guo J, Chowdhury A, Zhao M, Lindell E et al (2022) Iron chelator VLX600 inhibits mitochondrial respiration and promotes sensitization of neuroblastoma cells in nutrition-restricted conditions. Cancers (Basel) 14(13):3225. https://doi.org/10.3390/cancers14133225
Ekstrom TL, Pathoulas NM, Huehls AM, Kanakkanthara A, Karnitz LM (2021) VLX600 disrupts homologous recombination and synergizes with PARP inhibitors and cisplatin by inhibiting histone lysine demethylases. Mol Cancer Ther 20(9):1561–1571. https://doi.org/10.1158/1535-7163.MCT-20-1099
Kanakkanthara A, Kurmi K, Ekstrom TL, Hou X, Purfeerst ER, Heinzen EP et al (2019) BRCA1 deficiency upregulates NNMT, which reprograms metabolism and sensitizes ovarian cancer cells to mitochondrial metabolic targeting agents. Cancer Res 79(23):5920–5929. https://doi.org/10.1158/0008-5472.CAN-19-1405
Zhang X, Fryknäs M, Hernlund E, Fayad W, De Milito A, Olofsson MH et al (2014) Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun 5(1):3295. https://doi.org/10.1038/ncomms4295
Vitiello GA, Medina BD, Zeng S, Bowler TG, Zhang JQ, Loo JK et al (2018) Mitochondrial inhibition augments the efficacy of imatinib by resetting the metabolic phenotype of gastrointestinal stromal tumor. Clin Cancer Res 24(4):972–984. https://doi.org/10.1158/1078-0432.CCR-17-2697
Fryknas M, Zhang X, Bremberg U, Senkowski W, Olofsson MH, Brandt P et al (2016) Iron chelators target both proliferating and quiescent cancer cells. Sci Rep 6:38343. https://doi.org/10.1038/srep38343
Mody K, Mansfield AS, Vemireddy L, Nygren P, Gulbo J, Borad M (2019) A phase I study of the safety and tolerability of VLX600, an iron chelator, in patients with refractory advanced solid tumors. Invest New Drugs 37(4):684–692. https://doi.org/10.1007/s10637-018-0703-9
Zhang W, Lun S, Wang S-H, Jiang X-W, Yang F, Tang J et al (2018) Identification of novel coumestan derivatives as polyketide synthase 13 inhibitors against Mycobacterium tuberculosis. J Med Chem 61(3):791–803. https://doi.org/10.1021/acs.jmedchem.7b01319
Dong Y, Fu R, Chen J, Zhang K, Ji M, Wang M et al (2021) Discovery of benzocyclic sulfone derivatives as potent CXCR2 antagonists for cancer immunotherapy. J Med Chem 64(22):16626–16640. https://doi.org/10.1021/acs.jmedchem.1c01219
Chen C, Lu T, Chen P, Li Z, Yang Y, Fan S et al (2023) Cyclization strategy leads to highly potent Bromodomain and extra-terminal (BET) Bromodomain inhibitors for the treatment of acute liver injury. Eur J Med Chem 247:115023. https://doi.org/10.1016/j.ejmech.2022.115023
Wang Y, Kun H, Gao Y, Yuan D, Ling L, Liu J et al (2022) Discovery of quinazoline derivatives as novel small-molecule inhibitors targeting the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) interaction. Eur J Med Chem 229:113998. https://doi.org/10.1016/j.ejmech.2021.113998
Kumari R, Jat P (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 9:645593. https://doi.org/10.3389/fcell.2021.645593
Ingham M, Schwartz GK (2017) Cell-cycle therapeutics come of age. J Clin Oncol 35(25):2949–2959. https://doi.org/10.1200/jco.2016.69.0032
Plati J, Bucur O, Khosravi-Far R (2011) Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb) 3(4):279–296. https://doi.org/10.1039/c0ib00144a
Burke PJ (2017) Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer 3(12):857–870. https://doi.org/10.1016/j.trecan.2017.10.006
Carneiro BA, El-Deiry WS (2020) Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7):395–417. https://doi.org/10.1038/s41571-020-0341-y
Coombs GS, Schmitt AA, Canning CA, Alok A, Low IC, Banerjee N et al (2012) Modulation of Wnt/beta-catenin signaling and proliferation by a ferrous iron chelator with therapeutic efficacy in genetically engineered mouse models of cancer. Oncogene 31(2):213–225. https://doi.org/10.1038/onc.2011.228
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 14:48. https://doi.org/10.1186/s12943-015-0321
Acknowledgements
This work was supported by the Fujian Provincial Health Technology Project (2020QNA060, China), the Fujian Province Natural Science Foundation (2021J01309, China), Quanzhou City Science and Technology Program (2022NS007, China) and the Startup Fund for scientific research, Huaqiao University (20BS113, China). Thank the Instrumental Analysis Center of Huaqiao University.
Author information
Authors and Affiliations
Contributions
HL and YG wrote the main manuscript text; YX and XN synthesized target compounds; PZ and CW conducted activity tests; DT and HL prepared figures; XW prepared NMR data of intermediates and IR data for target compounds; JM designed target compounds, checked the data and corrected the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Gao, Y., Ni, X. et al. Design, synthesis and biological evaluation of 5H-[1,2,4]triazino[5,6-b]indole derivatives bearing a pyridinocycloalkyl moiety as iron chelators. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10840-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10840-w