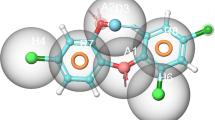
Abstract
Acinetobacter baumannii belongs to the ESKAPE family of pathogens and is a multi-drug resistant, gram-negative bacteria which follows the anaerobic form of respiration. A. baumannii is known to be the causative agent of hospital-related infections such as pneumonia, meningitis, endocarditis, septicaemia and a plethora of infections such as urinary tract infections found primarily in immunocompromised patients. These attributes of A. baumannii make it a priority pathogen against which potential therapeutic agents need to be developed. A. baumannii employs the formation of a biofilm to insulate its colonies from the outer environment, which allows it to grow under harsh environmental conditions and develop resistance against various drug molecules. Acyl-homoserine lactone synthase (AHLS) is an enzyme involved in the quorum-sensing pathway in A. baumannii, which is responsible for the synthesis of signal molecules known as acyl-homoserine lactones, which trigger the signalling pathway to regulate the factors involved in biofilm formation and regulation. The present study utilised a homology-modelled structure of AHLS to virtually screen it against the ZINC in trial/FDA-approved drug molecule library to find a subset of potential lead candidates. These molecules were then filtered based on Lipinski’s, toxicological and ADME properties, binding affinity, and interaction patterns to delineate lead molecules. Finally, three promising molecules were selected, and their estimated binding affinity values were corroborated using AutoDock 4.2. The identified molecules and a control molecule were subsequently subjected to MD simulations to mimic the physiological conditions of protein ligand-binding interaction under the influence of a GROMOS forcefield. The global and essential dynamics analyses and MM/PBSA based binding free energy computations suggested Droperidol and Cipargamin as potential inhibitors against the binding site of AHLS from A. baumannii. The binding free energy calculations based on the MM/PBSA method showed excellent results for Droperidol (− 50.02 ± 4.67 kcal/mol) and Cipargamin (− 42.29 ± 4.05 kcal/mol).
Graphical abstract













Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism and excretion
- DCCM:
-
Dynamical cross-correlation matrix
- FEL:
-
Free energy landscape
- MD:
-
Molecular dynamics
- MM/PBSA:
-
Molecular mechanics/Poisson–Boltzmann surface area
References
Howard A, O’Donoghue M, Feeney A, Sleator RD (2012) Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250. https://doi.org/10.4161/viru.19700
Al-Anazi KA, Abdalhamid B, Alshibani Z, Awad K, Alzayed A, Hassan H, Alsayiegh M (2012) Acinetobacter baumannii septicemia in a recipient of an allogeneic hematopoietic stem cell transplantation. Case Rep Transpl 2012:646195. https://doi.org/10.1155/2012/646195
Ni S, Li S, Yang N, Zhang S, Hu D, Li Q, Lu M (2015) Post-neurosurgical meningitis caused by Acinetobacter baumannii: case series and review of the literature. Int J Clin Exp Med 8:21833–21838
Chaari A, Mnif B, Bahloul M, Mahjoubi F, Chtara K, Turki O, Gharbi N, Chelly H, Hammami A, Bouaziz M (2013) Acinetobacter baumannii ventilator-associated pneumonia: epidemiology, clinical characteristics, and prognosis factors. Int J Infect Dis 17:e1225-1228. https://doi.org/10.1016/j.ijid.2013.07.014
Hartzell JD, Kim AS, Kortepeter MG, Moran KA (2007) Acinetobacter pneumonia: a review. MedGenMed 9:4
Rao RS, Karthika RU, Singh SP, Shashikala P, Kanungo R, Jayachandran S, Prashanth K (2008) Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol 26:333–337. https://doi.org/10.4103/0255-0857.43566
Daniels R, Vanderleyden J, Michiels J (2004) Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev 28:261–289. https://doi.org/10.1016/j.femsre.2003.09.004
Atkinson S, Chang CY, Sockett RE, Camara M, Williams P (2006) Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility. J Bacteriol 188:1451–1461. https://doi.org/10.1128/JB.188.4.1451-1461.2006
Lade H, Paul D, Kweon JH (2014) N-acyl homoserine lactone-mediated quorum sensing with special reference to use of quorum quenching bacteria in membrane biofouling control. Biomed Res Int 2014:162584. https://doi.org/10.1155/2014/162584
Saipriya K, Swathi CH, Ratnakar KS, Sritharan V (2020) Quorum-sensing system in Acinetobacter baumannii: a potential target for new drug development. J Appl Microbiol 128:15–27. https://doi.org/10.1111/jam.14330
Bindu Subhadra MHO, Choi CH (2016) Quorum sensing in Acinetobacter: with special emphasis on antibiotic resistance, biofilm formation and quorum quenching. AIMS Microbiol 2:27–41. https://doi.org/10.3934/microbiol.2016.1.27
Jha RK, Jabeer Khan R, Singh E, Kumar A, Jain M, Muthukumaran J, Singh AK (2022) An extensive computational study to identify potential inhibitors of Acyl-homoserine-lactone synthase from Acinetobacter baumannii (strain AYE). J Mol Graph Model 114:108168. https://doi.org/10.1016/j.jmgm.2022.108168
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K (2009) Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 77(Suppl 9):114–122. https://doi.org/10.1002/prot.22570
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Sander T, Freyss J, von Korff M, Rufener C (2015) DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model 55:460–473. https://doi.org/10.1021/ci500588j
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Chung J, Goo E, Yu S, Choi O, Lee J, Kim J, Kim H, Igarashi J, Suga H, Moon JS et al (2011) Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc Natl Acad Sci U S A 108:12089–12094. https://doi.org/10.1073/pnas.1103165108
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
BIOVIA DS (2019) Discovery studio visualization Dassault Systèmes BIOVIA
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible, and free. 26:1701–1718. https://doi.org/10.1002/jcc.20291
Huang W, Lin Z, van Gunsteren WF (2011) Validation of the GROMOS 54A7 force field with respect to beta-peptide folding. J Chem Theory Comput 7:1237–1243. https://doi.org/10.1021/ct100747y
Schuttelkopf AW, van Aalten DM (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D 60:1355–1363. https://doi.org/10.1107/S0907444904011679
Grant BJ, Rodrigues AP, ElSawy KM, McCammon JA, Caves LS (2006) Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22:2695–2696. https://doi.org/10.1093/bioinformatics/btl461
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461. https://doi.org/10.1517/17460441.2015.1032936
Kumari R, Kumar R, Lynn A (2014) g_mmpbsa–a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54:1951–1962. https://doi.org/10.1021/ci500020m
Chen J, Sawyer N, Regan L (2013) Protein-protein interactions: general trends in the relationship between binding affinity and interfacial buried surface area. Protein Sci 22:510–515. https://doi.org/10.1002/pro.2230
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637. https://doi.org/10.1002/bip.360221211
Chakravarty D, Guharoy M, Robert CH, Chakrabarti P, Janin J (2013) Reassessing buried surface areas in protein-protein complexes. Protein Sci 22:1453–1457. https://doi.org/10.1002/pro.2330
Barchuk WT, Salapatek AM, Ge T, D’Angelo P, Liu X (2013) A proof-of-concept study of the effect of a novel H3-receptor antagonist in allergen-induced nasal congestion. J Allergy Clin Immunol 132(838–846):e831-836. https://doi.org/10.1016/j.jaci.2013.05.001
Logan J, Carruthers NI, Letavic MA, Sands S, Jiang X, Shea C, Muench L, Xu Y, Carter P, King P et al (2012) Blockade of the brain histamine H3 receptor by JNJ-39220675: preclinical PET studies with [11C]GSK189254 in anesthetized baboon. Psychopharmacology 223:447–455. https://doi.org/10.1007/s00213-012-2733-x
Hien TT, White NJ, Thuy-Nhien NT, Hoa NT, Thuan PD, Tarning J, Nosten F, Magnusson B, Jain JP, Hamed K (2017) Estimation of the in vivo MIC of cipargamin in uncomplicated plasmodium falciparum malaria. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01940-16
Bouwman SA, Zoleko-Manego R, Renner KC, Schmitt EK, Mombo-Ngoma G, Grobusch MP (2020) The early preclinical and clinical development of cipargamin (KAE609), a novel antimalarial compound. Travel Med Infect Dis 36:101765. https://doi.org/10.1016/j.tmaid.2020.101765
Hirata I, Iwamoto M, Matsui H, Yoshinuma H, Funakoshi R (2020) Droperidol reduces postoperative nausea and vomiting and supports the continuation of intravenous patient-controlled analgesia with fentanyl. J Pharm Pharm Sci 23:220–230. https://doi.org/10.18433/jpps30902
Schaub I, Lysakowski C, Elia N, Tramer MR (2012) Low-dose droperidol (</=1 mg or </=15 mug kg-1) for the prevention of postoperative nausea and vomiting in adults: quantitative systematic review of randomised controlled trials. Eur J Anaesthesiol 29:286–294. https://doi.org/10.1097/EJA.0b013e328352813f
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. https://doi.org/10.1038/nrmicro1789
Cornejo-Juarez P, Cevallos MA, Castro-Jaimes S, Castillo-Ramirez S, Velazquez-Acosta C, Martinez-Oliva D, Perez-Oseguera A, Rivera-Buendia F, Volkow-Fernandez P (2020) High mortality in an outbreak of multidrug resistant Acinetobacter baumannii infection introduced to an oncological hospital by a patient transferred from a general hospital. PLoS ONE 15:e0234684. https://doi.org/10.1371/journal.pone.0234684
Acknowledgements
Dr. Amit Kumar Singh thanks Indian Council of Medical Research (ICMR) and Indian National Science Academy (INSA), New Delhi, India. The authors thank Sharda University for its support.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Dr. AKS Dr. JM. Data curation: Mr. RKJ, Mr. ES, Mr. RJK and Mr. AK. Formal analysis: Mr. RKJ, Mr. ES, Mr. RJK and Mr. AK. Investigation: Dr. AKS and Dr. JM. Methodology: Mr. RKJ, Mr. ES, Mr. RJK and Dr. MJ. Resources: Software: RKJ, Mr. ES, Mr. RJK and Dr. MJ. Supervision: Dr. AKS and Dr. JM. Validation: Dr. AKS, Dr. JM and Dr. MJ Visualization: RKJ, Mr. ES and Mr. RJK Roles/Writing—original draft—Mr. RKJ, Mr. ES, Mr. RJK and Mr. AK. Writing—review and editing—Mr. RKJ, Mr. ES, Mr. RJK, Mr. AK, Dr. MJ, Dr. JM and Dr. AKS.
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jha, R.K., Singh, E., Khan, R.J. et al. Droperidol as a potential inhibitor of acyl-homoserine lactone synthase from A. baumannii: insights from virtual screening, MD simulations and MM/PBSA calculations. Mol Divers 27, 1979–1999 (2023). https://doi.org/10.1007/s11030-022-10533-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10533-2