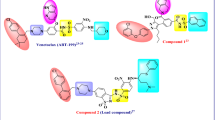
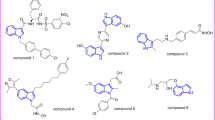
Abstract
Bcl-2, an anti-apoptotic protein, is a well-known and appealing cancer therapy target. Novel series of benzimidazole derivatives were synthesized and tested for their activity as Bcl-2 inhibitors on T98G glioblastoma, PC3 prostate, MCF-7 breast, and H69AR lung cancer cells. MTT assay was used to evaluate the cytotoxic effect. PI Annexin V Apoptosis Detection Kit was used to detect apoptosis. Expression levels of the Bcl-2 protein were examined by the Western blot analysis and qRT-PCR. All synthesized benzimidazole derivatives exhibited a cytotoxic effect on cancer cells with IC50 values in the range of 25.2–88.2 µg/mL. Among all derivatives, compounds C1 and D1 demonstrated a higher cytotoxic effect on cancer cells with IC50 values < 50 µg/mL, while a lower cytotoxic effect against human embryonic kidney cells with IC50 values of > 100 µg/mL. C1 and D1 caused a significant increase in the percentage of apoptotic cells in all types of cancer cell cells and both Bcl-2 mRNA and protein levels were significantly reduced. These results suggest that the novel benzimidazole derivatives may be candidates for apoptosis-inducing agents in cancer treatment by targeting anti-Bcl-2 proteins in cancer cells.
Graphical abstract





Similar content being viewed by others
References
Atmaca H, İlhan S, Batır MB et al (2020) Novel benzimidazole derivatives: synthesis, in vitro cytotoxicity, apoptosis and cell cycle studies. Chem Biol Interact 327:109163. https://doi.org/10.1016/j.cbi.2020.109163
Sridhar Goud N, Pooladanda V, Muni Chandra K et al (2020) Novel benzimidazole-triazole hybrids as apoptosis inducing agents in lung cancer: design, synthesis, 18F-radiolabeling & galectin-1 inhibition studies. Bioorg Chem 102:104125. https://doi.org/10.1016/j.bioorg.2020.104125
Perini GF, Ribeiro GN, Pinto Neto JV et al (2018) BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol 11:1–15. https://doi.org/10.1186/s13045-018-0608-2
Chen K, Chu BZ, Liu F et al (2015) New benzimidazole acridine derivative induces human colon cancer cell apoptosis in vitro via the ROS-JNK signaling pathway. Acta Pharmacol Sin 36:1074–1084. https://doi.org/10.1038/aps.2015.44
Vogler M (2014) Targeting BCL2-proteins for the treatment of solid tumours. Adv Med 2014:1–14. https://doi.org/10.1155/2014/943648
Kang MH, Reynolds CP (2009) BcI-2 Inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15:1126–1132. https://doi.org/10.1158/1078-0432.CCR-08-0144
Leber B, Geng F, Kale J, Andrews DW (2010) Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Rev Mol Med 12:1–19. https://doi.org/10.1017/S1462399410001572
Campbell KJ, Tait SWG (2018) Targeting BCL-2 regulated apoptosis in cancer. Open Biol 8(5):180002
Ilhan S (2020) Essential oils from Vitex agnus castus L. leaves induces caspase-dependent apoptosis of human multidrug-resistant lung carcinoma cells through intrinsic and extrinsic pathways. Nutr Cancer. https://doi.org/10.1080/01635581.2020.1823439
Zugazagoitia J, Guedes C, Ponce S et al (2016) Current challenges in cancer treatment. Clin Ther 38:1551–1566. https://doi.org/10.1016/j.clinthera.2016.03.026
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin. https://doi.org/10.3322/caac.21590
Mostaghni F, Taat F (2020) CoFe2O4as green and efficient catalyst for synthesis of multisubstituted imidazoles. Eurasian Chem Commun. https://doi.org/10.33945/SAMI/ECC.2020.4.1
Hakimi F, Fallah-Mehrjardi M, Golrasan E (2020) Yttrium aluminum garnet (YAG: Al5Y3O12) as an efficient catalyst for the synthesis of benzimidazole and benzoxazole derivatives. Chem Methodol 4:234–244
Rehan TA, Al-Lami N, Alanee RS (2021) Anti-cancer and antioxidant activities of some new synthesized 3-secondary amine derivatives bearing imidazo [1,2-A] pyrimidine. Eurasian Chem Commun 3:339–351
Haddazadeh E, Mohammadi MK (2020) One-pot synthesize of phenyl phenanthro ımidazole derivatives catalyzed by lewis acid in the presence of ammonium acetate. Chem Methodol 4:324–332
El Rashedy A, Aboul-Enein H (2013) Benzimidazole derivatives as potential anticancer agents. Mini-Reviews Med Chem 13:399–407. https://doi.org/10.2174/1389557511313030008
Yadav S, Narasimhan B, Kaur H (2016) Perspectives of benzimidazole derivatives as anticancer agents in the new era. Anticancer Agents Med Chem 16:1403–1425. https://doi.org/10.2174/1871520616666151103113412
Shinde VS, Lawande PP, Sontakke VA, Khan A (2020) Synthesis of benzimidazole nucleosides and their anticancer activity. Carbohydr Res 498:108178. https://doi.org/10.1016/j.carres.2020.108178
Shrivastava N, Naim MJ, Alam MJ et al (2017) Benzimidazole scaffold as anticancer agent: synthetic approaches and structure-activity relationship. Arch Pharm (Weinheim) 350:1–80. https://doi.org/10.1002/ardp.201700040
Henderson JP, Byun J, Williams MV et al (2001) Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Natl Acad Sci U S A 98:1631–1636. https://doi.org/10.1073/pnas.98.4.1631
Hładoń B, Gośliński T, Laskowska H et al (2002) In vitro cytostatic activity of 8-substituted and tricyclic analogues of acyclovir. Pol J Pharmacol 54:45–53
Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:1–14. https://doi.org/10.1186/1756-9966-30-87
International BMR (2020) Retracted: apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2020:2451249. https://doi.org/10.1155/2020/2451249
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilhan, S., Çamli Pulat, Ç., Oguz, F. et al. Design and synthesis of benzimidazole derivatives as apoptosis-inducing agents by targeting Bcl-2 protein. Mol Divers 27, 1703–1712 (2023). https://doi.org/10.1007/s11030-022-10524-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10524-3