Abstract
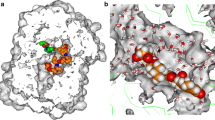
The involvement of Trypanosoma congolense sialidase alongside phospholipase A2 has been widely accepted as the major contributing factor to anemia during African animal trypanosomiasis. The enzymes aid the parasite in scavenging sialic acid and fatty acids necessary for survival in the infected host, but there are no specific drug candidates against the two enzymes. This study investigated the inhibitory effects of β-sitosterol on the partially purified T. congolense sialidase and phospholipase A2. Purification of the enzymes using DEAE cellulose column led to fractions with highest specific activities of 8016.41 and 39.26 µmol/min/mg for sialidase and phospholipase A2, respectively. Inhibition kinetics studies showed that β-sitosterol is non-competitive and an uncompetitive inhibitor of sialidase and phospholipase A2 with inhibition binding constants of 0.368 and 0.549 µM, respectively. Molecular docking of the compound revealed binding energies of − 8.0 and − 8.6 kcal/mol against the sialidase and phospholipase A2, respectively. Furthermore, 100 ns molecular dynamics simulation using GROMACS revealed stable interaction of β-sitosterol with both enzymes. Hydrogen bond interactions between the ligand and Glu284 and Leu102 residues of the sialidase and phospholipase A2, respectively, were found to be the major stabilizing forces. In conclusion, β-sitosterol could serve as a dual inhibitor of T. congolense sialidase and phospholipase A2; hence, the compound could be exploited further in the search for newer trypanocides.
Graphical abstract








Similar content being viewed by others
References
Cayla M, Rojas F, Silvester E, Venter F, Matthews KR (2019) African trypanosomes. Parasites Vectors 12(1):190. https://doi.org/10.1186/s13071-019-3355-5
Tchamdja E, Clausen PH, Kulo AE, Batawui K, Bauer B, Den Abbeele JV, Delespaux V, Hoppenheit A (2019) How rational drug use reduces trypanosome infections in cattle in chemo-resistance hot-spot villages of northern Togo. Acta Trop 190:159–165. https://doi.org/10.1016/j.actatropica.2018.11.023
McCulloch R, Cobbold CA, Figueiredo L, Jackson A, Morrison LJ, Mugnier MR, Papavasiliou N, Schnaufer A, Matthews K (2017) Emerging challenges in understanding trypanosome antigenic variation. Emerg Top Life Sci 1(6):585–592. https://doi.org/10.1042/ETLS20170104
Onyilagha C, Uzonna JE (2019) Host immune responses and immune evasion strategies in African trypanosomiasis. Front Immunol 10:2738. https://doi.org/10.3389/fimmu.2019.02738
Rathore NS, Manuja A, Manuja BK, Choudhary S (2016) Chemotherapeutic approaches against Trypanosoma evansi: retrospective analysis, current status and future outlook. Curr Top Med Chem 16(20):2316–2327. https://doi.org/10.2174/1568026616666160413125802
Silva Pereira S, Trindade S, De Niz M, Figueiredo LM (2019) Tissue tropism in parasitic diseases. Open Biol 9(5):190036. https://doi.org/10.1098/rsob.190036
Berninger M, Schmidt I, Ponte-Sucre A, Holzgrabe U (2017) Novel lead compounds in pre-clinical development against African sleeping sickness. MedChemComm 8(10):1872–1890. https://doi.org/10.1039/c7md00280g
Ibrahim MA, Musa AM, Aliyu AB, Mayaki HS, Gideon A, Islam MS (2013) Phenolics-rich fraction of Khaya senegalensis stem bark: antitrypanosomal activity and amelioration of some parasite-induced pathological changes. Pharm Biol 51(7):906–913. https://doi.org/10.3109/13880209.2013.771191
Ibrahim MA, Isah MB, Salman AA (2016) Antioxidant therapy against trypanosome infections: a review update. Curr Top Med Chem 16(20):2233–2244. https://doi.org/10.2174/1568026616666160413125622
Igbokwe IO (2018) Evolving anti-disease strategies from biochemical pathogenesis of african trypanosomiasis. Adv Cytol Pathol 3(2):33–39. https://doi.org/10.15406/acp.2018.03.00048
Mbaya A, Hussein Kumshe H, Nwosu CO (2012) The mechanisms of anaemia in trypanosomosis: a review. In: D. Silverberg (ed), Anemia, InTech. http://www.intechopen.com/books/anemia/the-mechanisms-of-anaemia-in-trypanosomosis-a-review. Accessed 9 Feb 2022
Habila N, Inuwa MH, Aimola IA, Udeh MU, Haruna E (2012) Pathogenic mechanisms of Trypanosoma evansi infections. Res Vet Sci 93(1):13–17. https://doi.org/10.1016/j.rvsc.2011.08.011
Balogun EO, Balogun JB, Yusuf S, Inuwa HM, Ndams IS, Sheridan P, Inaoka DK, Shiba T, Harada S, Kita K, Esievo KA, Nok AJ (2014) Anemia amelioration by lactose infusion during trypanosomosis could be associated with erythrocytes membrane de-galactosylation. Vet Parasitol 199(3–4):259–263. https://doi.org/10.1016/j.vetpar.2013.10.013
Juge N, Tailford L, Owen CD (2016) Sialidases from gut bacteria: a mini-review. Biochem Soc Trans 44(1):166–175. https://doi.org/10.1042/BST20150226
Aminu R, Ibrahim MA, Rahman MA, Dash R, Umar IA (2017) Trypanosuppresive effects of ellagic acid and amelioration of the trypanosome-associated pathological features coupled with inhibitory effects on trypanosomal sialidase in vitro and in silico. Phytomedicine 30:67–73. https://doi.org/10.1016/j.phymed.2017.04.013
Amaya MF, Watts AG, Damager I, Wehenkel A, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, Alzari PM (2004) Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure 12(5):775–784. https://doi.org/10.1016/j.str.2004.02.036
Gbem TT, Waespy M, Hesse B, Dietz F, Smith J, Chechet GD, Nok JA, Kelm S (2013) Biochemical diversity in the Trypanosoma congolense trans-sialidase family. PLoS Negl Trop Dis 7(12):e2549. https://doi.org/10.1371/journal.pntd.0002549
Aminu S, Ibrahim MA, Dada Chechet G, Onyike E (2022) Chemotherapeutic potentials of β-ionone against Trypanosoma congolense infection: Inhibition of parasite proliferation, anemia development, trans-sialidase (TconTS3 and TconTS4) gene expressions, and phospholipase A2. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.14048
Rossi MS, Boada-Sucre AA, Simoes MT, Boher Y, Rodriguez P, Moreno M, de Ruiz ML, Marquez ML, Finol HJ, Sanoja C, Payares G (2017) Adhesion of Trypanosoma evansi to red blood cells (RBCs): implications in the pathogenesis of anaemia and evasion of immune system. Diagn Pathol 2(1):1–10
Shuaibu MN, Kanbara H, Yanagi T, Ameh DA, Bonire JJ, Nok AJ (2001) Phospholipase A2 from Trypanosoma brucei gambiense and Trypanosoma brucei brucei: inhibition by organotins. J Enzyme Inhib 16(5):433–441. https://doi.org/10.1080/14756360109162392
Smith TK, Bringaud F, Nolan DP, Figueiredo LM (2017) Metabolic reprogramming during the Trypanosoma brucei life cycle. F1000Research. https://doi.org/10.12688/f1000research.10342.2
Burke JE, Dennis EA (2009) Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50(Suppl):S237–S242. https://doi.org/10.1194/jlr.R800033-JLR200
Nok AJ, Esievo KA, Ibrahim S, Ukoha AI, Ikediobi CO (1993) Phospholipase A2 from Trypanosoma congolense: characterization and haematological properties. Cell Biochem Funct 11(2):125–130. https://doi.org/10.1002/cbf.290110208
Tiralongo E, Martensen I, Grötzinger J, Tiralongo J, Schauer R (2003) Trans-sialidase-like sequences from Trypanosoma congolense conserve most of the critical active site residues found in other trans-sialidases. Biol Chem 384(8):1203–1213. https://doi.org/10.1515/BC.2003.133
Buchini S, Buschiazzo A, Withers SG (2008) A new generation of specific Trypanosoma cruzi trans-sialidase inhibitors. Angew Chem Int Ed Engl 47(14):2700–2703. https://doi.org/10.1002/anie.200705435
Folmer F, Jaspars M, Schumacher M, Dicato M, Diederich M (2010) Marine natural products targeting phospholipases A2. Biochem Pharmacol 80(12):1793–1800. https://doi.org/10.1016/j.bcp.2010.08.024
Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111(10):6130–6185. https://doi.org/10.1021/cr200085w
Aminu R, Umar IA, Rahman MA, Ibrahim MA (2017) Stigmasterol retards the proliferation and pathological features of Trypanosoma congolense infection in rats and inhibits trypanosomal sialidase in vitro and in silico. Biomed Pharmacother 89:482–489. https://doi.org/10.1016/j.biopha.2017.02.068
Saad SB, Ibrahim MA, Jatau ID, Shuaibu MN (2019) Trypanostatic activity of geranylacetone: mitigation of Trypanosoma congolense-associated pathological pertubations and insight into the mechanism of anaemia amelioration using in vitro and in silico models. Exp Parasitol 201:49–56. https://doi.org/10.1016/j.exppara.2019.04.011
Saad SB, Ibrahim MA, Jatau ID, Shuaibu MN (2020) The therapeutic potential of phytol towards Trypanosoma congolense infection and the inhibitory effects against trypanosomal sialidase. Exp Parasitol 216:107943. https://doi.org/10.1016/j.exppara.2020.107943
Abdulrashid NI, Aminu S, Adamu RM, Tajuddeen N, Isah MB, Jatau ID, Aliyu AB, Simelane MBC, Onyike E, Ibrahim MA (2022) Phloroglucinol as a potential candidate against Trypanosoma congolense infection: insights from in vivo, in vitro, molecular docking and molecular dynamic simulation analyses. Molecules 27(2):469. https://doi.org/10.3390/molecules27020469
Chinnasamy S, Selvaraj G, Selvaraj C, Kaushik AC, Kaliamurthi S, Khan A, Singh SK, Wei DQ (2020) Combining in silico and in vitro approaches to identification of potent inhibitor against phospholipase A2 (PLA2). Int J Biol Macromol 144:53–66. https://doi.org/10.1016/j.ijbiomac.2019.12.091
Wu AH, Ruan W, Todd J, Lynch KL (2014) Biological variation of β-sitosterol, campesterol, and lathosterol as cholesterol absorption and synthesis biomarkers. Clin Chim Acta 430:43–47. https://doi.org/10.1016/j.cca.2013.12.040
Grosjean K, Mongrand S, Beney L, Simon-Plas F, Gerbeau-Pissot P (2015) Differential effect of plant lipids on membrane organization: specificities of phytosphingolipids and phytosterols. J Biol Chem 290(9):5810–5825. https://doi.org/10.1074/jbc.M114.598805
Ju YH, Clausen LM, Allred KF, Almada AL, Helferich WG (2004) Beta-sitosterol, beta-sitosterol glucoside, and a mixture of beta-sitosterol and beta-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic aice. J Nutr 134(5):1145–1151. https://doi.org/10.1093/jn/134.5.1145
Hoet S, Pieters L, Muccioli GG, Habib-Jiwan JL, Opperdoes FR, Quetin-Leclercq J (2007) Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J Nat Prod 70(8):1360–1363. https://doi.org/10.1021/np070038q
Buratai LB, Nok AJ, Ibrahim S, Umar IA, Esievo KA (2006) Characterization of sialidase from bloodstream forms of Trypanosoma vivax. Cell Biochem Funct 24(1):71–77. https://doi.org/10.1002/cbf.1189
Gomes A, Hannahpep DP (1999) A novel fibrinolytic peptide from the Indian King Cobra (Ophiophagus hannah) venom. Biochem Biophys Res Commun 266(2):488–491. https://doi.org/10.1006/bbrc.1999.1818
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. Methods Mol Biol 1263:243–250. https://doi.org/10.1007/978-1-4939-2269-7_19
Lindahl E, Hess B, van der Spoel D (2001) GROMACS 3.0: a package for molecular simulation and trajectory analysis. J Mol Mod 7:306–317
Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A (2005) H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res 33(Web Server issue):W368–W371. https://doi.org/10.1093/nar/gki464
Case DA, Cheatham TE 3rd, Darden T, Gohlke H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688. https://doi.org/10.1002/jcc.20290
Schmit JD, Kariyawasam NL, Needham V, Smith PE (2018) SLTCAP: a simple method for calculating the number of ions needed for MD simulation. J Chem Theory Comput 14(4):1823–1827. https://doi.org/10.1021/acs.jctc.7b01254
Sousa da Silva AW, Vranken WF (2012) ACPYPE—AnteChamber PYthon parser interfacE. BMC Res Notes 5:367. https://doi.org/10.1186/1756-0500-5-367
Nok AJ, Balogun EO (2003) A bloodstream Trypanosoma congolense sialidase could be involved in anemia during experimental trypanosomiasis. J Biochem 133(6):725–730. https://doi.org/10.1093/jb/mvg093
Alkali Y, Gana AK, Abdulkadir A, Nzelibe HC (2015) Trypanocidal efficacy of two indigeneousethanolic plant extracts (Mimosa pigra and Ipomoea asarifolia) against Trypanosoma evansi phospholipase A2 activity. J Acute Dis 4(1):28–31
Lara-Ramirez EE, López-Cedillo JC, Nogueda-Torres B, Kashif M, Garcia-Perez C, Bocanegra-Garcia V, Agusti R, Uhrig ML, Rivera G (2017) An in vitro and in vivo evaluation of new potential trans-sialidase inhibitors of Trypanosoma cruzi predicted by a computational drug repositioning method. Eur J Med Chem 132:249–261. https://doi.org/10.1016/j.ejmech.2017.03.063
Sobolev OV, Afonine PV, Moriarty NW, Hekkelman ML, Joosten RP, Perrakis A, Adams PD (2020) A global Ramachandran score identifies protein structures with unlikely stereochemistry. Structure 28(11):1249-1258.e2. https://doi.org/10.1016/j.str.2020.08.005
Cournia Z, Allen B, Sherman W (2017) Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J Chem Inf Model 57(12):2911–2937. https://doi.org/10.1021/acs.jcim.7b00564
Al-Karmalawy AA, Dahab MA, Metwaly AM, Elhady SS, Elkaeed EB, Eissa IH, Darwish KM (2021) Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Front Chem 9:661230. https://doi.org/10.3389/fchem.2021.661230
Pal A, Levy Y (2019) Structure, stability and specificity of the binding of ssDNA and ssRNA with proteins. PLoS Comput Biol 15(4):e1006768. https://doi.org/10.1371/journal.pcbi.1006768
Nagasundaram N, Doss GP, Chakraborty C, Karthick V, Kumar D, Balaji V, Siva R, Aiping L, Zhang G, Hailong Z (2016) Mechanism of artemisinin resistance for malaria PfATP6 L263 mutations and discovering potential antimalarials: an integrated computational approach. Sci Rep 6:30106. https://doi.org/10.1038/srep30106
Alam S, Khan F (2018) Virtual screening, docking, ADMET and system pharmacology studies on Garciniacaged Xanthone derivatives for anticancer activity. Sci Rep 8(1):5524. https://doi.org/10.1038/s41598-018-23768-7
Van den Bergh CJ, Slotboom AJ, Verheij HM, de Haas GH (1989) The role of Asp-49 and other conserved amino acids in phospholipases A2 and their importance for enzymatic activity. J Cell Biochem 39(4):379–390. https://doi.org/10.1002/jcb.240390404
Eintracht J, Maathai R, Mellors A, Ruben L (1998) Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J 336(3):659–666. https://doi.org/10.1042/bj3360659
Danielson PB (2002) The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 3(6):561–597. https://doi.org/10.2174/1389200023337054
Manral C, Roy S, Singh M, Gautam S, Yadav RK, Rawat JK, Devi U, Ansari MN, Saeedan AS, Kaithwas G (2016) Effect of β-sitosterol against methyl nitrosourea-induced mammary gland carcinoma in albino rats. BMC Complement Altern Med 16:260. https://doi.org/10.1186/s12906-016-1243-5
Acknowledgements
We appreciate the management of Ahmadu Bello University, Zaria, Nigeria, for providing the enabling environment while carrying out the studies and a study fellowship to the first author. We also appreciate the support of the University of Groningen Peregrine for providing the computational resources for the molecular dynamic simulation studies. MAI is a recipient of the National Research Foundation Grant from TetFund, Nigeria (NRF2020/SETI/43). AUD is a beneficiary of The Ignacy Łukasiewicz Scholarship Programme from The Polish National Agency for Academic Exchange (NAWA).
Funding
Funding was provided by Tertiary Education Trust Fund.
Author information
Authors and Affiliations
Contributions
SA participated in the conceptualization of the study, methodology, resources, and initial draft of the manuscript. AUD wrote a section of the manuscript and performed the molecular dynamic simulation studies. ZAA was involved in formal analysis and editing. MWG was involved in formal analysis, investigation, writing—review, and editing. MAI contributed to the conceptualization of the study, methodology, formal analysis, data curation, supervision, writing—review, and editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aminu, S., Danazumi, A.U., Alhafiz, Z.A. et al. β-Sitosterol could serve as a dual inhibitor of Trypanosoma congolense sialidase and phospholipase A2: in vitro kinetic analyses and molecular dynamic simulations. Mol Divers 27, 1645–1660 (2023). https://doi.org/10.1007/s11030-022-10517-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10517-2