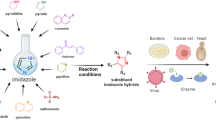
Abstract
The essential need for the potent anti-tubercular (anti-TB) agents with high selectivity and safety profile prompted us to synthesize a new series of quinazolinyl-bisspirooxindoles. The title compounds were synthesized by one-pot multicomponent [3 + 2] cycloaddition reaction under ultrasonication. Further, in vitro anti-TB activity was evaluated against Mycobacterium tuberculosis H37Rv. Among the screened compounds, two compounds (4q and 4x) showed potent activity with MIC value 1.56 µg/mL and four compounds exhibited significant activity (MIC = 3.125 µg/mL), and also cytotoxicity studies against RAW 264.7 cell lines reveal that most active compounds were less toxic to humans. In addition, in order to demonstrate the inhibitory properties, molecular docking studies were carried out and the results showed that the target compounds have good binding energy and better binding affinity within the active pocket, thus these compounds may consider to be as potent inhibitors toward selective targets.
Graphical Abstract








Similar content being viewed by others
References
Fogel N (2015) Tuberculosis: a disease without boundaries. Tuberculosis 95:527–531. https://doi.org/10.1016/j.tube.2015.05.017
WHO (2020) Global tuberculosis report. WHO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/. Accesed 14 Oct 2020
Bhowruth V, Dover LG, Besra GS (2007) 4 Tuberculosis chemotherapy: recent developments and future perspectives. Prog Med Chem 45:169–203. https://doi.org/10.1016/S0079-6468(06)45504-1
Du Preez I, Loots DT (2018) Novel insights into the pharmacometabonomics of first-line tuberculosis drugs relating to metabolism, mechanism of action and drug-resistance. Drug Metab Rev 50:466–481. https://doi.org/10.1080/03602532.2018.1559184
De Cock KM, Chaisson RE (1999) Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 3:457–465
Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, Soolingen D, Jensen P, Bayona J (2010) Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843. https://doi.org/10.1016/S0140-6736(10)60410-2
Muthukrishnan L (2021) Multidrug resistant tuberculosis—diagnostic challenges and its conquering by nanotechnology approach—an overview. Chem Biol Interact 337:109397. https://doi.org/10.1016/j.cbi.2021.109397
Zhou LM, Qu RY, Yang GF (2020) An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin Drug Discov 15:603–625. https://doi.org/10.1080/17460441.2020.1733526
Yu B, Yu Z, Qi PP, Yu DQ, Li HM (2015) Discovery of orally active anticancer candidate CFI-400945 derived from biologically promising spirooxindoles: success and challenges. Eur J Med Chem 95:35–40. https://doi.org/10.1016/j.ejmech.2015.03.020
Bora D, Kaushal A, Shankaraiah N (2021) Anticancer potential of spiro compounds in medicinal chemistry: a pentennial expedition. Eur J Med Chem 215:113263. https://doi.org/10.1016/j.ejmech.2021.113263
Haddad S, Boudriga S, Akhaja TN, Raval JP, Porzio F, Soldera A, Askri M, Knorr M, Rousselin Y, Kubickie MM, Rajani D (2015) A strategic approach to the synthesis of functionalized spirooxindole pyrrolidine derivatives: in vitro antibacterial, antifungal, antimalarial and antitubercular studies. N J Chem 39:520–528. https://doi.org/10.1039/c4nj01008f
Huang Y, Min W, Wu QW, Sun J, Shi DH, Yan CG (2018) Facile one-pot synthesis of spirooxindole-pyrrolidine derivatives and their antimicrobial and acetylcholinesterase inhibitory activities. N J Chem 42:16211–16216. https://doi.org/10.1039/C8NJ03813A
Al-Rashood ST, Hamed AR, Hassan GS, Alkahtani HM, Almehizia AA, Alharbi A, Al-Sanea MM, Eldehna WM (2020) Antitumor properties of certain spirooxindoles towards hepatocellular carcinoma endowed with antioxidant activity. J Enzyme Inhib Med Chem 35:831–839. https://doi.org/10.1080/14756366.2020.1743281
Nishtala VB, Mahesh C, Bhargavi G, Pasala VK, Basavoju S (2020) Synthesis of spirooxindolocarbamates based on Betti reaction: antibacterial, antifungal and antioxidant activities. Mol Divers 24:1139–1147. https://doi.org/10.1007/s11030-019-10017-w
Ye N, Chen H, Wold EA, Shi PY, Zhou J (2016) Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect Dis 2:382–392. https://doi.org/10.1021/acsinfecdis.6b00041
Chen L, Hao Y, Song H, Liu Y, Li Y, Zhang J, Wang Q (2020) Design, synthesis, characterization, and biological activities of novel spirooxindole analogues containing hydantoin, thiohydantoin, urea, and thiourea moieties. J Agric Food Chem 68:10618–10625. https://doi.org/10.1021/acs.jafc.0c04488
de Silva NH, Pyreddy S, Blanch EW, Hügel HM, Maniam S (2021) Microwave-assisted rapid synthesis of spirooxindole-pyrrolizidine analogues and their activity as anti-amyloidogenic agents. Bioorg Chem 114:105128. https://doi.org/10.1016/j.bioorg.2021.105128
Dai W, Jiang XL, Wu Q, Shi F, Tu SJ (2015) Diastereo- and enantioselective construction of 3, 3′-pyrrolidinyldispirooxindole framework via catalytic asymmetric 1, 3-dipolar cycloadditions. J Org Chem 80:5737–5744. https://doi.org/10.1021/acs.joc.5b00708
Rajesh SM, Perumal S, Menéndez JC, Yogeeswari P, Sriram D (2011) Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1, 3-dipolar cycloaddition. MedChemComm 2:626–630. https://doi.org/10.1039/c0md00239a
Mhiri C, Boudriga S, Askri M, Knorr M, Sriram D, Yogeeswari P, Nana F, Golz C, Strohmann C (2015) Design of novel dispirooxindolopyrrolidine and dispirooxindolopyrrolothiazole derivatives as potential antitubercular agents. Bioorg Med Chem Lett 25:4308–4313. https://doi.org/10.1016/j.bmcl.2015.07.069
Arumugam N, Almansour AI, Kumar RS, Krishna VS, Sriram D, Degec N (2021) Stereoselective synthesis and discovery of novel spirooxindolopyrrolidine engrafted indandione heterocyclic hybrids as antimycobacterial agents. Bioorg Chem 110:104798. https://doi.org/10.1016/j.bioorg.2021.104798
Zheng L, Wang H, Fan A, Li SM (2020) Oxepinamide F biosynthesis involves enzymatic d-aminoacyl epimerization, 3H-oxepin formation, and hydroxylation induced double bond migration. Nat Commun 11:1–10. https://doi.org/10.1038/s41467-020-18713-0
Wang CJ, Guo X, Zhai RQ, Sun C, Xiao G, Chen J, Wei MY, Shao CL, Gu Y (2021) Discovery of penipanoid C-inspired 2-(3, 4, 5-trimethoxybenzoyl)quinazolin-4(3H)-one derivatives as potential anticancer agents by inhibiting cell proliferation and inducing apoptosis in hepatocellular carcinoma cells. Eur J Med Chem 224:113671. https://doi.org/10.1016/j.ejmech.2021.113671
Qiu J, Chen W, Zhang Y, Zhou Q, Chen J, Li Y, Gao J, Gu X, Tang D (2019) Assessment of quinazolinone derivatives as novel non-nucleoside hepatitis B virus inhibitors. Eur J Med Chem 176:41–49. https://doi.org/10.1016/j.ejmech.2019.05.014
Monika SA, Suthar SK, Aggarwal V, Lee HB, Sharma M (2014) Synthesis of lantadene analogs with marked in vitro inhibition of lung adenocarcinoma and TNF-α induced nuclear factor-kappa B (NF-κB) activation. Bioorg Med Chem Lett 24:3814–3818. https://doi.org/10.1016/j.bmcl.2014.06.068
Plescia F, Maggio B, Daidone G, Raffa D (2021) 4-(3H)-quinazolinones N-3 substituted with a five membered heterocycle: a promising scaffold towards bioactive molecules. Eur J Med Chem 213:113070. https://doi.org/10.1016/j.ejmech.2020.113070
Shao LH, Fan SL, Meng YF, Gan YY, Shao WB, Wang ZC, Chen DP, Ouyang GP (2021) Design, synthesis, biological activities and 3D-QSAR studies of quinazolinone derivatives containing hydrazone structural units. N J Chem 45:4626–4631. https://doi.org/10.1039/d0nj05450j
Kamal A, Reddy BVS, Sridevi B, Ravikumar A, Venkateswarlu A, Sravanthi G, Sridevi JP, Yogeeswari P, Sriram D (2015) Synthesis and biological evaluation of phaitanthrin congeners as anti-mycobacterial agents. Bioorg Med Chem Lett 25:3867–3872. https://doi.org/10.1016/j.bmcl.2015.07.057
Lu W, Baig IA, Sun HJ, Cui CJ, Guo R, Jung IP, Wang D, Dong M, Yoon MY, Wang JG (2015) Synthesis, crystal structure and biological evaluation of substituted quinazolinone benzoates as novel antituberculosis agents targeting acetohydroxyacid synthase. Eur J Med Chem 94:298–305. https://doi.org/10.1016/j.ejmech.2015.03.014
Machado IV, dos Santos JRN, Januario MAP, Corrêa AG (2021) Greener organic synthetic methods: sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrason Sonochem 78:105704. https://doi.org/10.1016/j.ultsonch.2021.105704
Palanivel L, Gnanasambandam V (2020) Diversity oriented multi-component reaction (DOS-MCR) approach to access natural product analogues: regio- and chemo-selective synthesis of polyheterocyclic scaffolds: via one-pot cascade reactions. Org Biomol Chem 18:3082–3092. https://doi.org/10.1039/d0ob00368a
Allaka BS, Basavoju S, Gamidi RK (2021) A photoinduced multicomponent regioselective synthesis of 1, 4, 5-trisubstituted-1, 2, 3-triazoles: transition metal-, azide- and oxidant-free protocol. Adv Synth Catal 363:3560–3565. https://doi.org/10.1002/adsc.202100321
Allaka BS, Basavoju S, Gamidi RK (2022) Transition metal- and oxidant-free regioselective synthesis of 3, 4, 5-trisubstituted pyrazoles by means of [3 + 2] cycloaddition reactions. ChemistrySelect 7:3–7. https://doi.org/10.1002/slct.202200605
Ramesh P, Srinivasa Rao K, Trivedi R, Kumar BS, Prakasham RS, Sridhar B (2016) Highly efficient regio and diastereoselective synthesis of functionalized bis-spirooxindoles and their antibacterial properties. RSC Adv 6:26546–26552. https://doi.org/10.1039/C6RA00613B
Bhandari S, Sana S, Sridhar B, Shankaraiah N (2019) Microwave-assisted one-pot [3 + 2] cycloaddition of azomethine ylides and 3-alkenyl oxindoles: a facile approach to pyrrolidine-fused bis-spirooxindoles. ChemistrySelect 4:1727–1730. https://doi.org/10.1002/slct.201802847
Allaka BS, Basavoju S, Gamidi RK (2020) A green catalyst Fe(OTs)3/SiO2 for the synthesis of 4-pyrrolo-12-oxoquinazolines. ChemistrySelect 5:14721–14728. https://doi.org/10.1002/slct.202004012
Pogaku V, Krishna VS, Balachandran C, Rangan K, Sriram D, Aoki S, Basavoju S (2019) The design and green synthesis of novel benzotriazoloquinolinyl spirooxindolopyrrolizidines: antimycobacterial and antiproliferative studies. N J Chem 43:17511–17520. https://doi.org/10.1039/c9nj03802g
Pogaku V, Krishna VS, Sriram D, Rangan K, Basavoju S (2019) Ultrasonication-ionic liquid synergy for the synthesis of new potent anti-tuberculosis 1, 2, 4-triazol-1-yl-pyrazole based spirooxindolopyrrolizidines. Bioorg Med Chem Lett 29:1682–1687. https://doi.org/10.1016/j.bmcl.2019.04.026
Krishna VS, Zheng S, Rekha EM, Guddat LW, Sriram D (2019) Discovery and evaluation of novel mycobacterium tuberculosis ketol-acid reductoisomerase inhibitors as therapeutic drug leads. J Comput Aided Mol Des 33:357–366. https://doi.org/10.1007/s10822-019-00184-1
Van MJ, Kaspers GJ, Closs J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245. https://doi.org/10.1007/978-1-61779-080-5_20
Trott O, Olson AJ (2009) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Acknowledgements
The authors B. S. A. and S. B. thank the Director, NIT Warangal for providing the facilities. B. S. A. thanks the UGC New Delhi, India for providing Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allaka, B.S., Basavoju, S., Madhu Rekha, E. et al. Design and synthesis of novel quinazolinyl-bisspirooxindoles as potent anti-tubercular agents: an ultrasound-promoted methodology. Mol Divers 27, 1427–1436 (2023). https://doi.org/10.1007/s11030-022-10500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10500-x