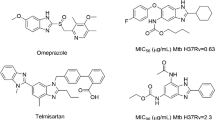
Abstract
Herein, we developed a convenient and efficient method via protonation of p-toluenesulfonic acid promoted cyclocondensation of o-phenylenediamine and aldehydes for selectively synthesizing 1,2-disubstituted benzimidazoles. This method displayed broad substrate adaptability and afforded the desired products in moderate to excellent yield in short reaction time. The effect of different substituents on the yield was investigated by extending optimum reaction conditions, which was further confirmed by theoretical calculations. It suggested that the surface electrostatic potential of oxygen atom and nitrogen atom on the substrates played important role in the synthesis of 1,2-disubstituted benzimidazoles. Besides, the crystal structure of compound 2t in the orthorhombic space group P2(1)/c was presented. Also, the anti-mycolicibacterium smegmatis (MC2155) activity was evaluated using rifampicin as a positive control. The products (2a, 2b, 2c, 2i, 2j, 2k, 2m) showed good antibacterial activities which were comparable to rifampicin.
Graphical abstract






Similar content being viewed by others
References
Akhtar W, Khan MF, Verma G, Shaquiquzzaman M, Rizvi MA, Mehdi SH, Akhter M, Alam MM (2016) Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur J Med Chem 126:705–753. https://doi.org/10.1016/j.ejmech.2016.12.010
Ntountaniotis D, Kellici T, Tzakos A, Kolokotroni P, Tselios T, Becker-Baldus J, Glaubitz C, Lin S, Makriyannis A, Mavromoustakos T (2014) The application of solid-state NMR spectroscopy to study candesartan cilexetil (TCV-116) membrane interactions. Comparative study with the AT1R antagonist drug olmesartan. Biophysica Acta (BBA) - Biomembranes 1838:2439–2450. https://doi.org/10.1016/j.bbamem.2014.06.003
Borba PAA, Pinotti M, Andrade GRS, Costa NB, Junior LRO, Fernandes D, Campos CEM, Stulzer HK (2015) The effect of mechanical grinding on the formation, crystalline changes and dissolution behaviour of the inclusion complex of telmisartan and β-cyclodextrins. Carbohydrate Polymer 133:373–383. https://doi.org/10.1016/j.carbpol.2015.06.098
Jakhar R, Paul S, Bhardwaj M, Kang SC (2016) Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett 372:89–100. https://doi.org/10.1016/j.canlet.2015.12.024
Garnock-Jones KP (2010) Bendamustine: a review of its use in the management of indolent non-Hodgkins lymphoma and mantle cell lymphoma. Drugs 70:1703–1718. https://doi.org/10.2165/11205860-000000000-00000
Hernández-Romero D, Rosete-Luna S, López-Monteon A, Chávez-Piña A, Pérez-Hernández N, Marroquín-Flores J, Cruz-Navarro A, Pesado-Gómez G, Morales-Morales D, Colorado-Peralta R (2021) First-row transition metal compounds containing benzimidazole ligands: An overview of their anticancer and antitumor activity. Coord Chem Rev 439:213930. https://doi.org/10.1016/j.ccr.2021.213930
Pinquier JL, Caplain H, Cabanis MJ, Dubruc C, Stalla-Bourdillon A, Rosenzweig P (2013) Inhibition of histamine-induced skin wheal and flare after 5 days of mizolastine. J Clin Pharmacol 36:72–78. https://doi.org/10.1002/j.1552-4604.1996.tb04154.x
Yurttas L, Demirayak S, Ciftci GA, Yildirim SU, Kaplancikli ZA (2013) Synthesis and biological evaluation of some 1,2-disubstituted benzimidazole derivatives as new potential anticancer agents. Archiv der Pharmazie (Weinheim) 346:403–414. https://doi.org/10.1002/ardp.201200452
Camacho J, Barazarte A, Gamboa N, Rodrigues J, Rojas R, Vaisberg A, Gilman R, Charris J (2011) Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial, cytotoxic and antitubercular agents. Bioorg Med Chem 19:2023–2029. https://doi.org/10.1016/j.bmc.2011.01.050
Shin Y, Suchomel J, Cardozo M, Duquette J, He X, Henne K, Hu YL, Kelly RC, Mccarter JD, Mcgee LR (2016) Discovery, optimization, and in vivo evaluation of benzimidazole derivatives am-8508 and am-9635 as potent and selective pi3kδ inhibitors. J Med Chem acs 59:431–447. https://doi.org/10.1021/acs.jmedchem.5b01651
Kamil A, Akhtar S, Khan A, Farooq E, Nishan U, Uddin R, Farooq U (2016) Synthesis, structure–activity relationship and antinociceptive activities of some 2-(2′-pyridyl) benzimidazole derivatives. Med Chem Res 25:1216–1228. https://doi.org/10.1007/s00044-016-1560-8
Fang B, Zhou CH, Rao XC (2010) Synthesis and biological activities of novel amine-derived bis-azoles as potential antibacterial and antifungal agents. Eur J Med Chem 41:4388–4398. https://doi.org/10.1016/j.ejmech.2010.06.012
Göker H, Özden S, Yıldız S, Boykin DW (2005) Synthesis and potent antibacterial activity against MRSA of some novel 1,2-disubstituted-1H-benzimidazole-N-alkylated-5-carboxamidines. Eur J Med Chem 40:1062–1069. https://doi.org/10.1016/j.ejmech.2005.05.002
Evans BE, Rittle KE, Bock MG, Dipardo RM, Chang R (1989) Methods for drug discovery. Development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem 31:2235–2246. https://doi.org/10.1021/jm00120a002
Sánchez-Mora A, Valdés H, Ramírez-Apan MT, Nieto-Camacho A, Hernández-Ortega S, Canseco-González D, Morales-Morales D (2019) NHC-Ir(I) complexes derived from 5,6-dinitrobenzimidazole Synthesis, characterization and preliminary evaluation of their in vitro anticancer activity. Inorganica Chimica Acta 496:119061. https://doi.org/10.1016/j.ica.2019.119061
Aragón-Muriel A, Liscano-Martínez Y, Rufino-Felipe E, Morales-Morales D, Oñate-Garzón J, Polo-Cerón D (2020) Synthesis, biological evaluation and model membrane studies on metal complexes containing aromatic N. O-chelate ligands Heliyon 6:e04126. https://doi.org/10.1016/j.heliyon.2020.e04126
Valdes-García J, Viviano-Posadas AO, Rivera-Chávez J, Ramírez-Apana T, Martínez-Vargas S, Aguirre-Hernández E, German-Acacio JM, Morales-Morales D, Dorazco-González A (2022) Crystal structures and study of interaction mode of bis-benzimidazole-benzene derivatives with DNA. J Mol Struct 1249:131582. https://doi.org/10.1016/j.molstruc.2021.131582
Yoon YK, Ali MA, Wei AC, Choon TS, Ismail R (2015) Synthesis and evaluation of antimycobacterial activity of new benzimidazole aminoesters. Eur J Med Chem 93:614–624. https://doi.org/10.1016/j.ejmech.2013.06.025
Chandrasekera NS, Alling T, Bailey MA, Files M, Early JV, Ollinger J, Ovechkina Y, Masquelin T, Desai PV, Cramer JW, Hipskind PA, Odingo JO, Parish T (2015) Identification of phenoxyalkylbenzimidazoles with antitubercular activity. J Med Chem 58:7273–7285. https://doi.org/10.1021/acs.jmedchem.5b00546
López-Pestaña JM, Ávila-Rey MJ, Martín-Aranda RM (2002) Ultrasound-promoted N-alkylation of imidazole. Catalysis by solid-base, alkali-metal doped carbons. Green Chem 4:628–630. https://doi.org/10.1039/b208479c
Chakrabortya A, Debnathb S, Ghoshb T, Maitib DK, Majumdar S (2018) An efficient strategy for N-alkylation of benzimidazoles/imidazoles in SDS-aqueous basic medium and N-alkylation induced ring opening of benzimidazoles. Tetrahedron 74:5932–5941. https://doi.org/10.1016/j.tet.2018.08.029
Wang B, Smith PJ (2003) Synthesis of a terbenzimidazole topoisomerase I poison via iterative borinate ester couplings. Tetrahedron Lett 44:8967–8969. https://doi.org/10.1016/j.tetlet.2003.10.007
Martin D, Siamaki AR, Belecki K, Gupton BF (2014) A convergent approach to the total synthesis of telmisartan via a suzuki cross-coupling reaction between two functionalized benzimidazoles. J Org Chem 80:1915–1919. https://doi.org/10.1021/jo5025333
Thapa P, Palacios PM, Tran T, Pierce BS, Foss FW (2020) 1,2-Disubstituted benzimidazoles by the iron catalyzed cross-dehydrogenative-coupling of isomeric o -phenylenediamine substrates. J Org Chem 85:1991–2009. https://doi.org/10.1021/acs.joc.9b02714
Ma Y, Xiong R, Feng Y, Zhang X, Xiong Y (2020) Synthesis of 1,2-disubstituted benzimidazoles through DDQ-oxidized intramolecular dehydrogenative coupling of N N’-dialkyl o-phenylenediamines. Tetrahedron. https://doi.org/10.1016/j.tet.2020.131474
Nan Z, Anderson KW, Huang X, Nguyen HN, Buchwald SL (2007) A palladium-catalyzed regiospecific synthesis of N-aryl benzimidazoles. Angew Chem Int Ed 46:7509–7512. https://doi.org/10.1002/ange.200702542
Zou B, Yuan Q, Ma D (2007) Synthesis of 1,2-disubstituted benzimidazoles by a cu-catalyzed cascade aryl amination/condensation process. Angew Chem Int Ed 119:2652–2655. https://doi.org/10.1002/ange.200700071
Putta RR, Chun S, Lee SB, Oh DC, Hong S (2020) Iron-catalyzed acceptorless dehydrogenative coupling of alcohols with aromatic diamines: selective synthesis of 1,2-disubstituted benzimidazoles. Front Chem 8:429. https://doi.org/10.3389/fchem.2020.00429
Das K, Mondal A, Srimani D (2018) Selective synthesis of 2-substituted and 1,2-disubstituted benzimidazoles directly from aromatic diamines and alcohols catalyzed by molecularly defined nonphosphine manganese(i) complex. J Org Chem 83:9553–9560. https://doi.org/10.1021/acs.joc.8b01316
Cheng JW, Shan SG, Qi S (2013) Selective synthesis of 2-substituted and 1,2-disubstituted benzimidazoles. Adv Mater Res 830:219–221. https://doi.org/10.1021/acs.joc.8b01316
Jacob RG, Dutra LG, Radatz CS, Mendes SR, Perin G, Lenardão EJ (2009) Synthesis of 1,2-disubstitued benzimidazoles using SiO2/ZnCl2. Tetrahedron Lett 50:1495–1497. https://doi.org/10.1016/j.tetlet.2009.01.076
Natividad HC, Uranga JG, Mónica N, Antonio P, Wunderlin DA, Santiag AN (2016) Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. Beilstein J Org Chem 12:2410–2419. https://doi.org/10.3762/bjoc.12.235
Yadav JS, Reddy BVS, Premalatha K, Shankar KS (2008) Bismuth(III)-catalyzed rapid and highly efficient synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles in water. Can J Chem 86:124–128. https://doi.org/10.1139/v07-140
Krishnamurthy GN, Shashikala N (2009) Synthesis of ruthenium(II) carbonyl complexes with 2-monosubstituted and 1,2-disubstituted benzimidazoles. J Serb Chem Soc 74:1085–1096. https://doi.org/10.2298/jsc0910085k
Samanta PK, Banerjee R, Richards RM, Biswas P (2018) Mesoporous silica supported ytterbium as catalyst for synthesis of 1,2-disubstituted benzimidazoles and 2-substituted benzimidazoles. Appl Organomet Chem 32:e4507. https://doi.org/10.1002/aoc.4507
Sharma H, Kaur N, Singh N, Jang DO (2015) Synergetic catalytic effect of ionic liquids and ZnO nanoparticles on the selective synthesis of 1,2-disubstituted benzimidazoles using a ball-milling technique. Green Chem 17:4263–4270. https://doi.org/10.1039/c5gc00536a
Chebolu R, Kommi DN, Kumar D, Bollineni N, Chakraborti AK (2012) Hydrogen-bond-driven electrophilic activation for selectivity control: scope and limitations of fluorous alcohol-promoted selective formation of 1,2-disubstituted benzimidazoles and mechanistic insight for rationale of selectivity. J Org Chem 77:10158. https://doi.org/10.1021/jo301793z
Santra S, Majee A, Hajra A (2012) Nano indium oxide: an efficient catalyst for the synthesis of 1,2-disubstituted benzimidazoles in aqueous media. Tetrahedron Lett 53:1974–1977. https://doi.org/10.1016/j.tetlet.2012.02.021
Bahrami K, Khodaei MM, Nejati A (2010) Synthesis of 1,2-disubstituted benzimidazoles, 2-substituted benzimidazoles and 2-substituted benzothiazoles in SDS micellesElectronic supplementary information (ESI) available: 1H NMR spectra for selected compounds. Green Chem 12:1237–1241. https://doi.org/10.1016/10.1039/c000047g
Azarifar D, Pirhayati M, Maleki B, Sanginabadi M, Yami RN (2010) Acetic acid-promoted condensation of o-phenylenediamine with aldehydes into 2-aryl-1-(arylmethyl)-1H-benzimidazoles under microwave irradiation. J Serb Chem Soc 75:1181–1189. https://doi.org/10.2298/JSC090901096A
Sahiba N, Agarwal DK, Manhas A, Sethiya A, Soni J, Jha PC, Agarwal S (2020) Mechanochemical approach for the selective synthesis of 1,2-disubstituted benzimidazoles and their molecular docking studies. Polycyclic Aromat Compd 26:1–19. https://doi.org/10.1080/10406638.2020.1768565
Saha D, Saha A, Ranu BC (2009) Remarkable influence of substituent in ionic liquid in control of reaction: simple, efficient and hazardous organic solvent free procedure for the synthesis of 2-aryl benzimidazoles promoted by ionic liquid, [pmim]BF4. Green Chem 11:733–737. https://doi.org/10.1039/B823543K
Milania JLS, Bezerraa WdA, Valdoa AKSM, Martinsa FT, Camargob LTFM, Carvalho-Silvab VH, Santosc SS, Cangussua D, Chagas RP (2019) Zinc complexes with 1,2-disubstituted benzimidazole ligands: experimental and theoretical studies in the catalytic cycloaddition of CO2 with epoxides. Polyhedron 173:114134. https://doi.org/10.1016/j.poly.2019.114134
Anary-Abbasinejad M, Mosslemin MH, Hassanabadi A, Safa ST (2010) p-Toluene sulfonic acid–catalyzed, solvent-free synthesis of symmetrical bisamides by reaction between aldehydes and amides. Synth Commun 40:2209–2214. https://doi.org/10.1080/00397910903219617
Shi Q, Dou GL (2010) Efficient synthesis of quinoxaline derivatives catalyzed by p-toluenesulfonic acid under solvent-free conditions. Synth Commun 38:3329–3337. https://doi.org/10.1080/00397910802136664
Jin T, Zhang S, Li T (2002) p-Toluenesulfonic acid-catalyzed efficient synthesis of dihydropyrimidines: improved high yielding protocol for the biginelli reaction. Synth Commun 32:1847–1851. https://doi.org/10.1081/SCC-120004068
Mohebat R, Simin N, Yazdani-Elah-Abadi A (2017) A rapid and highly efficient microwave-promoted four-component domino reaction for the synthesis of novel spiro[benzo[a]chromeno[2,3-c]phenazine] derivatives under solvent-free conditions. Polycyclic Aromat Compd 39:148–158. https://doi.org/10.1080/10406638.2017.1293698
Vidavalur S, Gajula MB, Tadikonda R, Nakka M, Dega S, Yadav SK, Voosala C (2014) PTSA catalyzed straightforward protocol for the synthesis of 2-(N-acyl)aminobenzimidazoles and 2-(N-acyl)aminobenzothiazoles in PEG. Tetrahedron Lett 55:2691–2694. https://doi.org/10.1016/j.tetlet.2014.03.040
Vallés-García C, Cabrero-Antonino M, Navalón S, Álvaro M, Dhakshinamoorthy A, García H (2019) Nitro functionalized chromium terephthalate metal-organic framework as multifunctional solid acid for the synthesis of benzimidazoles. J Colloid Interface Sci 560:885–893. https://doi.org/10.1016/j.jcis.2019.10.093
Lin S, Yang L (2005) A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett 46:4315–4319. https://doi.org/10.1002/chin.200540137
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdiscip Rev Comput Molecular Sci 1:153–163. https://doi.org/10.1002/wcms.19
Murray JS, Brinck T, Lane P, Paulsen K, Politzer P (1994) Statistically-based interaction indices derived from molecular surface electrostatic potentials: A general interaction properties function (GIPF). J Mol Struct (Thoechem) 307:55–64. https://doi.org/10.1016/0166-1280(94)80117-7
Frisch MJ, Trucks GW and Schlegel HB, et al (2016) Gaussian 16, Revision A.03, Gaussian, Inc.: Wallingford CT.
Tian L, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Lu T, Chen F (2012) Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J Mol Graph Model 38:314–323. https://doi.org/10.1016/j.jmgm.2012.07.004
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) Properties of atoms in molecules: atomic volumes. J Am Chem Soc 109:7968–7979. https://doi.org/10.1021/ja00260a006
Nguyen TV, Peszko MT, Melander RJ, Melander C (2019) Using 2-aminobenzimidazole derivatives to inhibit Mycobacterium smegmatis biofilm formation. MedChemComm 10:456–459. https://doi.org/10.1039/c9md00025a
Yoon YK, Ali MA, Choon TS, Ismail R, Wei AC, Kumar RS, Osman H, Beevi F (2013) Antituberculosis: synthesis and antimycobacterial activity of novel benzimidazole derivatives. Biomed Res Int 10:926309. https://doi.org/10.1155/2013/926309
Acknowledgements
We are grateful to Dr C. Y. Wang for NMR spectra, Dr Z. L. Wei for Ms spectra and Dr L. Ye for single-crystal X-ray structure determination.
Author information
Authors and Affiliations
Contributions
JF performed the synthesis of experiments, analyzed the date, the theoretical calculation of ESP and wrote the original draft. YY performed the antimicrobial activity experiments. KL and SW performed the synthesis of some compounds. FL designed and guided antimicrobial activity experiments. YZ optimized geometry configurations of aldehyde. QS performed the analysis of single-crystal X-ray structure. QG designed some experiments. YZ conceived and designed the experiments, explained experimental phenomena and laws, and writing–review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, J., Yue, Y., Liu, K. et al. PTSA-catalyzed selective synthesis and antibacterial evaluation of 1,2-disubstituted benzimidazoles. Mol Divers 27, 873–887 (2023). https://doi.org/10.1007/s11030-022-10460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10460-2