Abstract
A novel green protocol has been developed for the synthesis of quinazolinone–tetrazole conjugates (7a–g, 8a–g and 9a–g) using recyclable nano-CuFe2O3 catalyst in water. Initially, 2-mercapto-3-substituted phenethylquinazolin-4(3H)-one (5a–c) was prepared by using nano-CuFe2O3 catalyst in water. Then, compounds (5a–c) were reacted with 1-bromo-3-chloropropane under nano-CuFe2O3 catalyst in water solvent to give S-alkylated quinazolinone core intermediate (6a–c), which was subsequently reacted with 1-substituted-1H-tetrazole-5-thiol (2a-g) by employing the similar reaction conditions to afford the final target compounds. The regioselective formation of C–S bond was unambiguously confirmed by single-crystal X-ray diffraction. The anti-cancer activity of the derivatives on various cancer cell lines such as SIHA, MD-AMB-231 and HepG2 was evaluated. Remarkably, compounds, 7f, 8f, 9a, 9d and 9f, showed potent activity in MD-AMB-231 cancer cell line (IC50: 9.13–10.3 µM), while the same derivatives showed significant potent activity in SiHa and HepG2 cancer cell lines (IC50: 17.46–27.0 µM). Most significantly, compound 7o (IC50: 8.15 µM) showed potent activity, compared to the drug etoposide (IC50: 18.11 µM) against MD-AMB-231 cell line. Flow cytometry analysis revealed that compounds 7f, 8f, 9a, 9d and 9f arrested the cell growth in the G1 phase in MD-AMB-231 cell line.
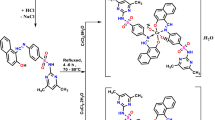
Graphical abstract













Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Loo TW, Clarke DM (2000) Blockage of drug resistance in vitro by disulfiram, a drug used to treat alcoholism. J Nat Can Ins 92:898–902. https://doi.org/10.1093/jnci/92.11.898
Perez CA, Brady LW (1997) Principles and practice of radiation oncology. Lippincott-Raven Publisher, Philadelphia
Despaigne AA, Parrilha GL, Izidoro JB, da Costa PR, dos Santos RG, Piro OE, Castellano EE, RochaBeraldo WRH (2012) 2-Acetylpyridine- and 2-benzoylpyridine-derived hydrazones and their gallium(III) complexes are highly cytotoxic to glioma cells. Eur J Med Chem 50:163–172. https://doi.org/10.1016/j.ejmech.2012.01.051
Lu Y, Li C, Wang Z, Ross CR, Chen J, Dalton T, Li W, Miller DD (2009) Discovery of 4-substituted methoxybenzoyl-aryl-thiazole as novel anticancer agents: synthesis, biological evaluation, and structure−activity relationships. J Med Chem 52:1701–1711. https://doi.org/10.1021/jm801449a
Bonola G, Da Re P, Magistretti MJ, Massarani E, Setnikar I (1968) 1-Aminoacyl-2,3-dihydro-4(1H)-quinazolinone derivatives with choleretic and antifibrillatory activity. J Med Chem 11:1136–1139. https://doi.org/10.1021/jm00312a007
Okumura K, Oine T, Yamada Y, Hayashi G, Nakama M (1968) 4-Oxo-1,2,3,4-tetrahydroquinazolines. I. Syntheses and pharmacological properties of 2-methyl-3-aryl-4-oxo-1,2,3,4-tetrahydroquinazolines and their 1-acyl derivatives. J Med Chem 11:348–352. https://doi.org/10.1021/jm00308a036
VanRyzin RJ, Trpold JH (1980) the toxicology profile of the anti-inflammatory drug proquazone in animals. Drug Chem Toxicol 3:361–379. https://doi.org/10.3109/01480548009030126
Hughes AN, Rafi I, Griffin MJ et al (1999) Phase I studies with the nonclassical antifolate nolatrexed dihydrochloride (AG337, THYMITAQ) administered orally for 5 days. Clin Cancer Res 5(1):111–118
Widemann BC, Balis FM, Godwin KS, McCully C, Adamson PC (1999) The plasma pharmacokinetics and cerebrospinal fluid penetration of the thymidylate synthase inhibitor raltitrexed (Tomudex) in a nonhuman primate model. Cancer Chemother Pharmacol 44(6):439–443. https://doi.org/10.1007/s002800051116
Mani Chandrica P, Yakaiah T, Raghu Ram Rao A, Narsaiah B, ChakraReddy N, Sridhar V, VenkateshwaraRao J (2008) Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur J Med Chem 43:846–852. https://doi.org/10.1016/j.ejmech.2007.06.010
Hyao S, Mvera MJ, Strycker W, Leipzi T, Klup R, Hartzler H (1965) New Sedative and Hypotensive 3-Substituted 2,4(1H,3H)-Quinazolinediones. J Med Chem 8:807–811. https://doi.org/10.1021/jm00330a017
Daniel BY, Jason WG, Stephanie LN, Arely VP, Matthew TC, David AB (2006) Anti-inflammatory activity in skin by biomimetic of Evodia rutaecarpa extract from traditional Chinese medicine. J Dermato Sci 42:13–21. https://doi.org/10.1016/j.jdermsci.2005.12.009
Archana VK, Srivastava C, Ramesh K, Ashok G (2002) Synthesis of potential quinazolinonyl pyrazolines and quinazolinyl isoxazolines as anticonv ulsant agents. Indian J Chem 41B:2371–2375
Yen MH, Sheu JR, Peng IH, Lee YM, Chern JW (1996) Pharmacological activity of DC-015, a novel potent and selective alpha 1-adrenoceptor antagonist. J Pharm Pharmacol 48(1):90–95. https://doi.org/10.1111/j.2042-7158.1996.tb05884.x
Chan HJ, Hong JS, Kuyper LF, Baccanari DP, Joyner SS, Tansik RL, Boytos CM, Rudolph SK (1995) Selective inhibitors of Candida albicans dihydrofolate reductase: activity and selectivity of 5-(arylthio)-2,4-diaminoquinazolines. J Med Chem 38:3608–3616. https://doi.org/10.1021/jm00018a021
Al-Omary FAM, Abou-Zeid LA, Nagi MN, Habib EE, Abdel-Aziz AA-M, El-Azab AS, Abdel-Hamide SG, Al-Omar MA, Al-Obaid AM, El-Subbagh HI (2010) Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2,6-substituted-quinazolin-4-ones. Bioorg Med Chem 18:2849–2863. https://doi.org/10.1016/j.bmc.2010.03.019
Li J, Meng Y, Liu Y, Feng Z-Q, Chen X-G (2010) A quinazoline derivative, exhibits high potent antitumor activity against human gynecologic malignancies. Invest New Drugs 28:132–138. https://doi.org/10.1007/s10637-009-9225-9
Chandregowda V, Kush AK, Reddy GC (2009) Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur J Med Chem 44:3046–3055. https://doi.org/10.1016/j.ejmech.2008.07.023
Foote KM, Mortlock AA, Heron NM, Jung FH, Hill GB, Pasquet G, Brady MC, Green S, Heaton SP, Kearney S, Keen NJ, Odedra R, Wedge SR, Wilkinson RW (2008) Synthesis and SAR of 1-acetanilide-4-aminopyrazole-substituted quinazolines: selective inhibitors of Aurora B kinase with potent anti-tumor activity. Bioorg Med Chem Lett 18:1904–1909. https://doi.org/10.1016/j.bmcl.2008.02.002
Wang D, Gao F (2013) Quinazoline derivatives: synthesis and bioactivities. Chem Cent J 7:95. https://doi.org/10.1186/1752-153X-7-95
Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT (2005) Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett 15:1915–1917. https://doi.org/10.1016/j.bmcl.2005.01.083
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH (2002) ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 26:5749–5754
Al-Rashood ST, Aboldaha IA, Nagi MN, Abou-Zeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Youssef KM, Al-Obaid AM, Subbagh HI (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem 14:8608–8621. https://doi.org/10.1016/j.bmc.2006.08.030
Griffin RJ, Srinivasan S, Bowman K, Calvert AH, Curtin NJ, Newell DR, Pemberton LC, Golding BT (1998) Resistance-modifying agents. Synthesis and biological properties of quinazolinone inhibitors of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP). J Med Chem 41:5247–5256. https://doi.org/10.1021/jm980273t
Butler RN, Katritzky AR, Rees CW, Scriven EFV (1996) Comprehensive heterocyclic chemistry II. Elsevier, London
da Silva FMC, dos Santos JC, Campos JLO, Mafud AC, Polikarpov I, Figueira ACM (2013) Structure-based Identification of novel PPAR gamma ligands. Bioorg Med Chem Lett 23:5795–5802. https://doi.org/10.1016/j.bmcl.2013.09.010
Shemyakina OA, Mal’kina AG, Albanov AI, Trofimov BA (2011) Regio- and stereodirection of addition of tetrazole to α, β-acetylenic γ-hydroxy nitrile: synthesis of 1- and 2-(Z)-(2-cyanoethenyl- 1-hydroxyalkyl)tetrazoles. Chem Heterocycl Compd 47:464–469. https://doi.org/10.1007/s10593-011-0782-4
Kumar CNSSP, Parida DK, Santhoshi A, Kota AK, Sridhar B, Rao V (2011) Synthesis and biological evaluation of tetrazole containing compounds as possible anticancer agents. Med Chem Commun 2:486–492. https://doi.org/10.3390/ijms21113965
Dileep K, Polepalli S, Jain N, Buddana SK, Prakasham RS, Murty MSR (2018) Synthesis of novel tetrazole containing hybrid ciprofloxacin and pipemidic acid analogues and preliminary biological evaluation of their antibacterial and antiproliferative activity. Mol Divers 22(1):83–93. https://doi.org/10.1007/s11030-017-9795-y
Kommula D, Polepalli S, Jain N, Murty MSR (2018) Synthesis and preliminary antiproliferative activity of novel 4-substituted phenylsulfonyl piperazines with tetrazole moiety. Indian J Pharm Sci 80(5):930–939. https://doi.org/10.4172/pharmaceutical-sciences.1000440
Kommula D, Mohana Rao K, Ramalingeswara Rao B, Vishnu Vardhan MVPS, Ramakrishna S, Jagadeesh Babu N, Murty MSR (2017) Regioselective synthesis and preliminary cytotoxic activity properties of tetrazole appendage N-substituted piperazine derivatives. Org Commun 10(3):178–189. https://doi.org/10.25135/acg.oc.20.17.04.018
Serp P, Philippot K (eds) (2013) Nanomaterials in catalysis. Wiley, Weinheim, Germany
Shylesh S, Thiel W (2010) Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 49:3259–3428. https://doi.org/10.1002/anie.200905684
Shahrzad A, Zinatossadat H (2019) Fe3O4 MNPs as a green catalyst for syntheses of functionalized [1,3]-oxazole and 1H-pyrrolo-[1,3]-oxazole derivatives and evaluation of their antioxidant activity. Mol Divers 23:885–896. https://doi.org/10.1007/s11030-019-09916-9
Fatemeh CF, Shahrzad A, Reza KK (2020) A PANI-Fe3O4@ZnO nanocomposite: a magnetically separable and applicable catalyst for the synthesis of chromeno-pyrido[d]pyrimidine derivatives. RSC Adv 10:15614–15621. https://doi.org/10.1039/D0RA01978J
Abu-Dief AM, Abdel-Fatah SM (2018) Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ J Basic Appli Sci 7:55–67. https://doi.org/10.1016/j.bjbas.2017.05.008
Beletskaya IP, Ananikov VP (2011) Transition-metal-catalyzed C−S, C−Se, and C−Te bond formation via cross-coupling and atom-economic addition reactions. Chem Rev 111:1596–1636. https://doi.org/10.1021/cr100347k
Rabindranath S, Sailesh C, Dhiraj B, Biswajit S, Pranab G (2022) Environmentally benign approach towards C-S cross-coupling reaction by organo-copper(II) complex. Mol divers 26:505–511. https://doi.org/10.1007/s11030-020-10180-5
Shiri P, Amani AM, Aboonajmi J (2021) Supported Cu(II)-Schiff base: novel heterogeneous catalyst with extremely high activity for eco-friendly, one-pot and multi-component C-S bond-forming reaction toward a wide range of thioethers as biologically active cores. Mol divers. https://doi.org/10.1007/s11030-021-10227-1
Yavari I, Sirouspour M, Souri S (2010) One-pot synthesis of functionalized 4-oxo-2-thioxo-1,3-thiazinanes from primary amines, CS2, and itaconic anhydride. Mol divers 14:611–615. https://doi.org/10.1007/s11030-009-9194-0
Kondo T, Mitsudo TA (2000) Metal-catalyzed carbon−sulfur bond formation. Chem Rev 100:3205–3220. https://doi.org/10.1021/cr9902749
Manabe K, Iimura S, Sun XM, Kobayashi S (2002) Dehydration reactions in water. Brønsted acid−surfactant-combined catalyst for ester, ether, thioether, and dithioacetal formation in water. J Am Chem Soc 124:11971–11978. https://doi.org/10.1021/ja026241j
Tsukinoki T, Nagashima S, Mitoma Y, Tashiro M (2000) Organic reaction in water. Part 4. New synthesis of vicinal diamines using zinc powder-promoted carbon–carbon bond formation. Green Chem 2:117–119. https://doi.org/10.1039/B001533O
Murty MSR, Katiki MR, Rao BR, Babu NJ, Buddana SK, Prakasham RS (2014) Magnetically recyclable nano-Fe 2 O 3 -catalyzed chemoselective synthesis and antioxidant activity of diethyl (3-((5-Aryl-1H-1,2,4-triazol-3-yl)thio)propyl)phosphonates. Synth Commun 44:2724–2737. https://doi.org/10.1080/00397911.2014.918146
Murty MSR, Katiki MR, Kommula D (2016) Multicomponent click synthesis of β-hydroxy/benzyl 1,2,3-triazoles catalyzed by magnetically recyclable nano iron oxide in water. Can Chem Trans 4:47–61. https://doi.org/10.13179/canchemtrans.2016.04.01.0270
Rao KM, Dileep K, Polepalli S, Jain N, Murty MSR (2019) A one-pot multicomponent ‘Click’ approach to the synthesis of novel tamoxifen-triazole conjugates using nano iron oxide catalyst and their preliminary antiproliferative activity studies. Lett Drug Des Discov 16:846–860. https://doi.org/10.2174/1570180815666180621100314
Dileep K, Murty MSR (2017) Aqueous, one-pot, three-component reaction for efficient synthesis of 2-[4-(Arylsulfonyl)piperazin-1-yl]-1,3-benzothiazole, -1H-benzimidazole, or -1,3-benzoxazole Derivatives. Synlett 28:2295–2298. https://doi.org/10.1055/s-0036-1590972
Kommula D, Madugula SRM (2017) Synthesis of benzimidazoles/benzothiazoles by using recyclable, magnetically separable nano-Fe2O3 in aqueous medium. J Iran Chem Soc 14:1665–1671. https://doi.org/10.1007/s13738-017-1107-z
Uday KR, Dileep K, Harsha VRK, Nageswar YVD (2018) Microwave assisted amination of 2-chloro azoles with various substituted aryl piperazines and aryl sulfonylpiperazines under neat conditions. Curr Microwave Chem 5(1):62–72. https://doi.org/10.2174/2213335605666180227154226
Rao BR, Katiki MR, Dileep K, Sai SN, Ruby JA, Murty MSR (2019) Synthesis of novel benzamide- piperazine-sulfonamide hybrids as potential anticancer agents. Croat Chem Acta 92(3):393–402. https://doi.org/10.5562/cca3535
Pezhman S (2021) Novel hybrid molecules based on triazole-β-lactam as potential biological agents. Mini Rew Med Chem 21(5):536–553
Madasu C, Gudem S, Sistla R et al (2017) Synthesis and anti-inflammatory activity of some novel pyrimidine hybrids of myrrhanone A, a bicyclic triterpene of Commiphora mukul gum resin. Monatsh Chem 148:2183–2193. https://doi.org/10.1007/s00706-017-2024-7
Mallavadhani UV, Chandrashekhar M, Nayak VL et al (2015) Synthesis and anticancer activity of novel fused pyrimidine hybrids of myrrhanone C, a bicyclic triterpene of Commiphora mukul gum resin. Mol Divers 19:745–757. https://doi.org/10.1007/s11030-015-9621-3
Yonghai F, Congming Lu, Huijie W, Minjia M, Yunlei Z, Dewei R, Lei L, Hengbo Y (2010) Spinel copper–iron-oxide magnetic nanoparticles with cooperative Cu(i) and Cu(ii) sites for enhancing the catalytic transformation of 1,2-propanediol to lactic acid under anaerobic conditions. Catal Sci Technol 10:8094–8107. https://doi.org/10.1039/D0CY01733G
Chandrashekhar M, Mallavadhani NVL, UV, Sistla, R. (2016) Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: synthesis, cytotoxic activity and cell based studies. Eur J Med Chem 114:293–307. https://doi.org/10.1016/j.ejmech.2016.03.013
Mallavadhani UV, Chandrashekhar M, Shailaja K, Sistla R (2019) Design, synthesis, anti-inflammatory, cytotoxic and cell based studies of some novel side chain analogues of myrrhanones A & B isolated from the gum resin of Commiphora mukul. Bioorg Chem 82:306–323. https://doi.org/10.1016/j.bioorg.2018.10.039
Shang YH, Fan LY, Li XX, Liu MX (2015) Y(OTf)3-catalyzed heterocyclic formation via aerobic oxygenation: an approach to dihydro quinazolinones and quinazolinones. Chin Chem Lett 26:1355–1358. https://doi.org/10.1016/j.cclet.2015.07.026
Acknowledgements
The authors are thankful to the Director, Indian Institute of Chemical Technology, Hyderabad, for the encouragement, KD and GK are thankful to CSIR, New Delhi, India, for the award of research fellowships and thank Dr. Jagadeesh Babu Nanubolu for X‐ray analysis, Laboratory of X‐ray Crystallography. We thank CSIR for financial support under the 12th Five Year plan projects “Affordable Cancer Therapeutics (ACT)” (CSC 0301) and “Small Molecules in Lead Exploration (SMiLE)” (CSC0111).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kommula, D., Chintakunta, P.K., Garikapati, K. et al. Nano-CuFe2O3-catalyzed green synthesis of novel quinazolinone–tetrazole hybrids as anti-cancer agents. Mol Divers 27, 425–441 (2023). https://doi.org/10.1007/s11030-022-10432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10432-6