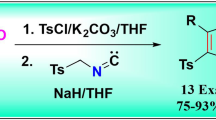
Abstract
A novel, convenient and efficient protocol to access functionalized 5-amidoimidazoles is developed via one-pot synthesis from readily available materials of arylamines, carbon disulfide and isocyanides. The transformation was realized at room temperature and provided 5-amidoimidazoles in moderate to good yields in the presence of NaH. In addition, control experiments indicated that the process might be achieved via the base-induced cyclization of activated methylene isocyanides with N,N-disubstituted thioureas that produced from the reaction of amines and carbon disulfide.



Similar content being viewed by others
References
Eicher T, Hauptmann S, Speicher A (2012) The chemistry of heterocycles: structure, reactions, synthesis, and applications, Third completely revised and enlarged edition, Wiley-VCH Verlag & Co. KGaA. Boschstr. 2:217–228
Zhang L, Peng X-M, Damu GLV, Gen R-X, Zhou C-H (2014) Comprehensive review in current developments of imidazole-based medicinal chemistry. Med Res Rev 34:340–437. https://doi.org/10.1002/med.21290
Wang X-Q, LiuL-X LY, Sun C-J, Chen W, Li L, Zhang H-B, Yang X-D (2013) design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur J Med Chem 62:111–121. https://doi.org/10.1016/j.ejmech.2012.12.040
Zarnowska ED, Rodgers FC, Oh I, Rau V, Lor C, Laha KT, Jurd R, Rudolph U, Eger EI 2nd, Pearce RA (2015) Etomidate blocks LTP and impairs learning but does not enhance tonic inhibition in mice carrying the N265M point mutation in the Beta3 subunit of the GABAA receptor. Neuropharmacology 93:171–178. https://doi.org/10.1016/j.neuropharm.2015.01.011
Leclercq L, Nardello-Rataj V (2016) Pickering emulsions based on cyclodextrins: a smart solution for antifungal azole derivatives topical delivery. Eur J Pharm Sci 82:126–137. https://doi.org/10.1016/j.ejps.2015.11.017
Viesselmann CW, Descourouez JL, Jorgenson MR, Radke NA, Odorico JS (2016) Clinically significant drug interaction between clotrimazole and tacrolimus in pancreas transplant recipients and associated risk of allograft rejection. Pharmacotherapy 36:335–341. https://doi.org/10.1002/phar.1718
Ríos-Malváez ZG, Cano-Herrera MA, Dávila-Becerril JC, Mondragón-Solórzano G, Ramírez-Apan MT, Morales-Morales D, Barroso-Flores J, Santillán-Benítez JG, Unnamatla MVB, García-Eleno MA, González-Rivas N, Cuevas-Yañez E (2021) Synthesis, characterization and cytotoxic activity evaluation of 4-(1,2,3-triazol-1-yl) salicylic acid derivatives. J Mol Struct 1225:129149. https://doi.org/10.1016/j.molstruc.2020.129149
Linares-Anaya O, Avila-Sorrosa A, Díaz-Cedillo F, Gil-Ruiz LÁ, Correa-Basurto J, Salazar-Mendoza D, Orjuela AL, Alí-Torres J, Ramírez-Apan MT, Morales-Morales D (2021) Synthesis, characterization, and preliminary in vitro cytotoxic evaluation of a series of 2-substituted benzo [d] [1,3] azoles. Molecules 26:2780. https://doi.org/10.3390/molecules26092780
Hernández-Romero D, Rosete-Luna S, López-Monteon A, Chávez-Piña A, Pérez-Hernández N, Marroquín-Flores J, Cruz-Navarro A, Pesado-Gómez G, Morales-Morales D, Colorado-Peralta R (2021) First-row transition metal compounds containing benzimidazole ligands: an overview of their anticancer and antitumor activity. Coord Chem Rev 439:213930. https://doi.org/10.1016/j.ccr.2021.213930
Peytam F, Adib M, Shourgeshty R, Mohammadi-Khanaposhtani M, Jahani M, Imanparast S, Faramarzi MA, Mahdavi M, Moghadamnia AA, Rastegar H, Larijani B (2020) Design and synthesis of new imidazo[1,2-b]pyrazole derivatives, in vitro α-glucosidase inhibition, kinetic and docking studies. Mol Divers 24:69–80. https://doi.org/10.1007/s11030-019-09925-8
Garrison JC, Youngs WJ (2005) Ag(I) N-heterocyclic carbene complexes: synthesis, structure, and application. Chem Rev 105:3978–4008. https://doi.org/10.1021/cr050004s
Flanigan DM, Romanov-Michailidis F, White NA, Rovis T (2015) Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem Rev 115:9307–9387. https://doi.org/10.1021/acs.chemrev.5b00060
D’Souza F, Smith PM, Zandler ME, McCarty AL, Itou M, Araki Y, Ito O (2004) Energy transfer followed by electron transfer in a supramolecular triad composed of boron dipyrrin, zinc porphyrin, and fullerene: a model for the photosynthetic antenna-reaction center complex. J Am Chem Soc 126:7898–7907. https://doi.org/10.1021/jă47u
Amarasekara AS (2016) Alkaloids and isoprenoids modification by copper(i) -catalyzed huisgen 1,3-dipolar cycloaddition (click chemistry): toward new functions and molecular architectures. Chem Rev 116:6133–6183. https://doi.org/10.1021/acs.chemrev.5b00302
Goossens K, Lava K, Bielawski CW, Binnemans K (2016) Ionic liquid crystals: versatile materials. Chem Rev 116:4643–4807. https://doi.org/10.1021/cr400334b
Wang W, Ji X, Kapur A, Zhang C, Mattoussi H (2015) A multifunctional polymer combining the imidazole and zwitterion motifs as a biocompatible compact coating for quantum dots. J Am Chem Soc 137:14158–14172. https://doi.org/10.1021/jacs.5b08915
Alves MJ, Booth BL, Fernanda M, Proenç JRP (1990) Synthesis of 5-amino-4-(cyanoformimidoyl)-1H-imidazole: a reactive intermediate for the synthesis of 6-carbamoyl-1,2-dihydropurines and 6-carbamoylpurines. J. Chem. Soc Perkin Trans 1:1705–1712. https://doi.org/10.1039/P19900001705
Schrimsher JL, Schendel FJ, Stubbe J (1986) Isolation of a multifunctional protein with aminoimidazole ribonucleotide synthetase, glycinamide ribonucleotide synthetase, and glycinamide ribonucleotide transformylase activities: characterization of aminoimidazole ribonucleotide synthetase. J Biochemistry 25:4356–4365. https://doi.org/10.1021/bi00363a027
Schrimsher JL, Schendel FJ, Stubbe J, Smith JM (1986) Purification and characterization of aminoimidazole ribonucleotide (AIR) synthetase from. Biochemistry 25:4366–4371. https://doi.org/10.1021/bi00363a028
Estramareix B, David S (1986) Biosynthesis of thiamine: origin of the methyl carbon atom of the pyrimidine moiety in salmonella typhimurium. Biochem Biophys Res Commun 134:1136–1141. https://doi.org/10.1016/0006-291X(86)90369-4
Ferris JP, Orgel LE (1966) An unusual photochemical rearrangement in the synthesis of adenine from hydrogen cyanide. J Am Chem Soc 88:1074–1074. https://doi.org/10.1021/ja00957a050
Koch TH, Rodehorst RM (1974) Quantitative investigation of the photochemical conversion of diaminomaleonitrile to diaminofumaronitrile and 4-amino-5-cyanoimidazole. J Am Chem Soc 96:6707–6710. https://doi.org/10.1002/chin.197452149
Reayi A, Hosmane RS (2004) Inhibition of adenosine deaminase by novel 5:7 fused heterocycles containing the imidazo[4,5-e][1,2,4]triazepine ring system: a structure-activity relationship study. J Med Chem 47:1044–1050. https://doi.org/10.1021/jm0304257
Samari HR, Seglen PO (1998) Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, andn 6-mercaptopurine riboside evidence for involvement of amp-activated protein kinase. J Biol Chem 273:23758–23763. https://doi.org/10.1074/jbc.273.37.23758
Kadir K, Shaw G, Wright D (1980) Purines, pyrimidines, and imidazoles. part 56. Some aminoimidazole-carboxamidines and derived adenines. J. Chem. Soc Perkin Trans 1:2728–2731. https://doi.org/10.1039/P19800002728
Shabalin DA, Dunsford JJ, Ngwerume S, Saunders AR, Gill DM, Camp JE (2020) Synthesis of 2,4-disubstituted imidazoles via nucleophilic catalysis. Synlett 31(08):797–800. https://doi.org/10.1055/s-0039-1690832
Ananthu S, Aneeja T, Anilkumar G (2021) N-Arylation of imidazoles: an overview. ChemistrySelect 6:9794–9805. https://doi.org/10.1002/slct.202102411
Li Y, Huang Z, Mo G, Jiang W, Zheng C, Feng P, Ruan Z (2021) Direct electrochemical synthesis of sulfur-containing triazolium inner salts. Chin J Chem 39:942–946. https://doi.org/10.1002/cjoc.202000586
Beuvin M, Manneveau M, Diab S, Picard B, Sanselme M, Piettre SR, Legros J, Chataigner I (2018) New synthesis of imidazole derivatives from cyanobenzenes. Tetrahedron Lett 59:4487–4491. https://doi.org/10.1016/j.tetlet.2018.11.020
Hitoshi O, Tomohiro A, Kazutada I, Makoto I, Jae-Hoon C, Hirokazu K, Toshiyuki K (2018) Synthesis of double-13C-labeled imidazole derivatives. Tetrahedron Lett 59(39):3516–3518. https://doi.org/10.1016/j.tetlet.2018.07.048
McLaughlin M, Mohareb RM, Rapoport H (2003) An efficient procedure for the preparation of 4-substituted 5-aminoimidazoles. J Org Chem 68:50–54. https://doi.org/10.1021/jo026257s
Hunt JT, Bartlett P (1978) Regioselective synthesis of 5-amino-4- imidazolecarboxylates via isonitrile cycloaddition. Synthesis. https://doi.org/10.1055/s-1978-24872
Dukanya D, Swaroop TR, Rangappa S, Rangappa KS, Basappa, (2019) Cyclization of activated methylene isocyanides with methyl N(N), N′- di(tri)substituted carbamimidothioate: a novel entry for the synthesis of n,1-aryl-4-tosyl/ethoxycarbonyl-1h-imidazol-5-amines. SynOpen 3:71–76. https://doi.org/10.1055/s-0039-1690328
Sapuppo G, Wang Q, Swinnen D, Zhu J (2014) Copper-catalyzed three- component synthesis of 5-acetamidoimidazoles from carbodiimides, acyl chlorides and isocyanides. Org Chem Front 1:240–246. https://doi.org/10.1039/c4qo00034j
Soh CH, Chui W-K, Lam Y-L (2006) Synthesis of 2,4-disubstituted 5-aminoimidazoles using microwave irradiation. J Comb Chem 8:464–468. https://doi.org/10.1021/cc060030j
Shaabani A, Mohammadian R, Afshari R, Hooshmand SE, Nazeri MT, Javanbakht S (2021) The status of isocyanide-based multi-component reactions in Iran (2010–2018). Mol Divers 25:1145–1210. https://doi.org/10.1007/s11030-020-10049-7
Mohammadi-Khanaposhtani M, Jalalimanesh N, Saeedi M, Larijani B, Mahdavi M (2020) Synthesis of highly functionalized organic compounds through Ugi post-transformations started from propiolic acids. Mol Divers 24:855–887. https://doi.org/10.1007/s11030-019-09975-y
Sisko J, Kassick AJ, Mellinger M, Filan JJ, Allen A, Olsen MA (2000) An investigation of imidazole and oxazole syntheses using aryl-substituted TosMIC reagents. J Org Chem 65:1516–1524. https://doi.org/10.1021/jo991782l
Acknowledgements
The work was financially supported by the National Innovation and Entrepreneurship Training Program for college students (No. 202010349031) and Natural Science Foundation for colleges and universities in Anhui Province (No. YJS20210138).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict to interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, J., Zhang, Y., Yan, Q. et al. NaH-promoted one-pot synthesis of 5-amidoimidazoles from arylamines, carbon disulfide and isocyanides. Mol Divers 27, 135–143 (2023). https://doi.org/10.1007/s11030-022-10413-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10413-9