Abstract
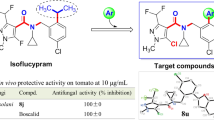
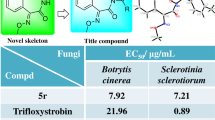
Inspired by the highly effective and broad-spectrum antifungal activity of ergosterol biosynthesis inhibitions, a series of novel 1,2,4-triazole derivatives containing oxime ether moiety were constructed for screening the bioactivity against phytopathogenic fungi. The (Z)- and (E)-isomers of target compounds were successfully separated and identified by the spectroscopy and single crystal X-ray diffraction analyses. The bioassay results showed that the (Z)-isomers of target compounds possessed higher antifungal activity than the (E)-isomers. Strikingly, the compound (Z)-5o exhibited excellent antifungal activity against Rhizoctonia solani with the EC50 value of 0.41 μg/mL in vitro and preventive effect of 94.58% in vivo at 200 μg/mL, which was comparable to the positive control tebuconazole. The scanning electron microscopy observation indicated that the compound (Z)-5o caused the mycelial morphology to become wizened and wrinkled. The molecular docking modes of (Z)-5o and (E)-5o with the potential target protein RsCYP51 were especially compared. And the main interactions between ligands and amino acid residues were carefully analyzed to preliminarily explain the mechanism leading to the difference of activity between two isomers. The study provided a new lead molecular skeleton for developing novel triazole fungicides targeting ergosterol biosynthesis.
Graphical abstract









Similar content being viewed by others
References
Wang Q, Tang B-H, Cao M-H (2020) Synthesis, characterization, and fungicidal activity of novel fangchinoline derivatives. Bioorg Med Chem 28:115778. https://doi.org/10.1016/j.bmc.2020.115778
Sheng T, Kong M-M, Wang Y-J, Wu H-J, Gu Q, Chuang AS, Li S-K, Gao X-W (2021) Discovery and preliminary mechanism of 1-carbamoyl β-carbolines as new antifungal candidates. Eur J Med Chem 222:113563. https://doi.org/10.1016/j.ejmech.2021.113563
Aggarwal R, Sumran G (2020) An insight on medicinal attributes of 1,2,4-triazoles. Eur J Med Chem 205:112652. https://doi.org/10.1016/j.ejmech.2020.112652
Xu H, Su X, Guo M-B, An R, Mou Y-H, Zhuang H, Guo C (2020) Design, synthesis, and biological evaluation of novel miconazole analogues containing selenium as potent antifungal agents. Eur J Med Chem 198:112360. https://doi.org/10.1016/j.ejmech.2020.112360
Foley DA, Callaghan YO, Brien NMO, Carthy FOM, Maguire AR (2010) Synthesis and characterization of stigmasterol oxidation products. J Agric Food Chem 58:1165–1173. https://doi.org/10.1021/jf9024745
Zhang J-X, Li L-P, Lv Q-Z, Yan L, Wang Y, Jiang Y-Y (2019) The Fungal CYP51s: Their functions, structures, related drug resistance, and inhibitors. Front Microbiol 10:691. https://doi.org/10.3389/fmicb.2019.00691
Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL (2015) Azole fungicides–understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci 71:1054–1058. https://doi.org/10.1002/ps.4029
Tyndall JDA, Sabherwal M, Sagatova AA, Keniya MV, Negroni J, Wilson RK, Woods MA, Tietjen K, Monk BC (2016) Structural and functional elucidation of yeast lanosterol 14α-demethylase in complex with agrochemical antifungals. PLoS ONE 1(12):e0167485. https://doi.org/10.1371/journal.pone.0167485
Fan X-Y, Fu Y, Nie Y-X, Matsumoto H, Wang Y, Hu T-T, Pan Q-Q, Lv T-X, Fang H-D, Xu H-R, Wang Y, Ge H, Zhu G-N, Liu Y-H, Wang Q-W, Wang M-C (2021) Keystone taxa-mediated bacteriome response shapes the resilience of the paddy ecosystem to fungicide triadimefon contamination. J Hazard Mater 417:126061. https://doi.org/10.1016/j.jhazmat.2021.126061
Macar TK, Macar O, Yalçιn M, Yalçιn E, Çavuşoğlu K (2021) Preventive efficiency of Cornelian cherry (Cornus mas L.) fruit extract in diniconazole fungicide-treated Allium cepa L. roots. Sci Rep 11:2534. https://doi.org/10.1038/s41598-021-82132-4
Cui N, Xu H-Y, Yao S-J, He Y-W, Zhang H-C, Yu Y-L (2018) Chiral triazole fungicide tebuconazole: enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ Sci Pollut Res 25:25468–25475. https://doi.org/10.1007/s11356-018-2587-9
Wang Y, Wang M-M, Xu L-T, Sun Y, Feng J-T (2020) Baseline sensitivity and toxic action of the sterol demethylation inhibitor flusilazole against botrytis cinerea. Plant Dis 104:2986–2993. https://doi.org/10.1094/PDIS-11-19-2478-RE
Mısır MN, Mısır G, Bekircan O, Kantekin H, Oztürk D, Durmus M (2021) Sulfur bridged new metal-free and metallo phthalocyanines carrying 1,2,4-triazole rings and their photophysicochemical properties. Polyhedron 207:115361. https://doi.org/10.1016/j.poly.2021.115361
Zhang Y, Damu GLV, Cui S-F, Mi J-L, Tangadanchu VKR, Zhou C-H (2017) Discovery of potential antifungal triazoles: design, synthesis, biological evaluation, and preliminary antifungal mechanism exploration. Med Chem Commun 8:1631. https://doi.org/10.1039/C7MD00112F
Su S-J, Chen M, Li Q, Wang Y-H, Chen S, Sun N, Xie C-W, Huai Z-Y, Huang Y-J, Xue W (2021) Novel penta-1,4-diene-3-one derivatives containing quinazoline and oxime ether fragments: design, synthesis and bioactivity. Bioorg Med Chem 32:115999. https://doi.org/10.1016/j.bmc.2021.115999
Han X, Lv W, Guo S-Y, Cushman M, Liang J-H (2015) Synthesis and structure–activity relationships of novel 9-oxime acylides with improved bactericidal activity. Bioorg Med Chem 23:6437–6453. https://doi.org/10.1016/j.bmc.2015.08.020
Koo SJ, Ahn SC, Lim JS, Chae SH, Kim JS, Lee JH, Cho JH (1997) Biological activity of the new herbicide LGC-40863 {benzophenone O-[2,6-bis[(4,6-dimethoxy-2-pyrimidinyl)oxy]benzoyl]oxime}. Pestic Sci 51:109–114. https://doi.org/10.1002/(SICI)1096-9063(199710)51:2<109::AID-PS585>3.0.CO;2-7
Bian Q, Zhao R-Q, Peng X-J, Gao L-J, Zhou G-N, Yu S-J, Zhao W-G (2021) Design, Synthesis, and fungicidal activities of novel piperidyl thiazole derivatives containing oxime ether or oxime ester moieties. J Agric Food Chem 69:3848–3858. https://doi.org/10.1021/acs.jafc.0c07581
Ouyang G, Cai X-J, Chen Z, Song B-A, Bhadury PS, Yang S, Jin L-H, Xue W, Hu D-Y, Song Z (2008) Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J Agric Food Chem 56:10160–10167. https://doi.org/10.1021/jf802489e
Fu C-R, Pei J, Ning Y, Liu M, Shan P-C, Liu J, Li Y-Q, Hu F-Z, Zhu Y-Q, Yang H-Z, Zou X-M (2014) Synthesis and insecticidal activities of novel pyrazole oxime ether derivatives with different substituted pyridyl rings. Pest Manag Sci 70:1207–1214. https://doi.org/10.1002/ps.3672
Tellier F, Fritz R, Kerhoas L, Ducrot P-H, Einhorn J, Sinclair AC, Leroux P (2008) Characterization of metabolites of fungicidal cymoxanil in a sensitive strain of botrytis cinerea. J Agric Food Chem 56:8050–8057. https://doi.org/10.1021/jf8010917
Nofiani R, Mattos-Shipley KD, Lebe K, Han L-C, Iqbal Z, Willis C, Simpson T, Cox R (2018) Strobilurin biosynthesis in Basidiomycete fungi. Nat Commun 9:3940. https://doi.org/10.1038/s41467-018-06202-4
Takagaki M, Kataoka S, Kida K, Miura I, Fukumoto S, Tamai R (2010) Disease-controlling effect of a novel fungicide pyribencarb against Botrytis cinerea. J Pestic Sci 35(1):10–14. https://doi.org/10.1584/jpestics.G09-24
Ravenzwaay BV, Akiyama M, Landsiede R, Kieczka H, Cunha G, Schneider S, Kaspers U, Kaufmann W, Osawa M (2007) Toxicological overview of a novel strobilurin fungicide, orysastrobin. J Pestic Sci 32:207. https://doi.org/10.1584/jpestics.G06-52
Reuveni M (2000) Efficacy of trifloxystrobin (Flint), a new strobilurin fungicide, in controlling powdery mildews on apple, mango and nectarine, and rust on prune trees. Crop Prot 19:335–341. https://doi.org/10.1016/S0261-2194(00)00026-0
Wang M-L, Du Y, Ling C, Yang Z-K, Jiang B-B, Duan H-X, An J, Li X-H, Yang X-L (2021) Design, synthesis and antifungal/anti-oomycete activity of pyrazolyl oxime ethers as novel potential succinate dehydrogenase inhibitors. Pest Manag Sci. https://doi.org/10.1002/ps.6418
Blessing B, Submuth R (1993) Stimulating effects in bacterial toxicity tests—a source of error in evaluating the risk posed by chemicals. Wat Res 27:225–229. https://doi.org/10.1016/0043-1354(93)90079-W
European Food Safety Authority (EFSA), Parma I (2014) Conclusion on the peer review of the pesticide risk assessment of the active substance fenhexamid. EFSA J 12(7):3744. https://doi.org/10.2903/j.efsa.2014.3744
Peng J-N, Wang K, Feng T-Y, Zhang H-Z, Li X-H, Qi Z-Q (2020) The effect (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the membrane damage mechanism. Molecules 25(1):94. https://doi.org/10.3390/molecules25010094
Wang C, Li Z-L, Ju Y-M, Koo S (2012) Mechanism and scope of the MnIII-initiated oxidation of β-ketocarbonyl compounds: furan synthesis. Eur J Org Chem 35:6976–6985. https://doi.org/10.1002/ejoc.201201194
Liu X-W, Ma J-M, Colson A-O, Doersen DC, Ebetino FH (2008) Synthesis and biological activity of novel peptide mimetics as melanocortin receptor agonists. Bioorg Med Chem Lett 18:1223–1228. https://doi.org/10.1016/j.bmcl.2007.11.109
Chen M, Geng C-W, Han L, Liu Y, Yu Y-K, Lu A-M, Yang C-L, Li G-H (2021) Design, synthesis, crystal structure, and herbicidal activity of novel pyrrolidine-2,4-dione derivatives incorporating an alkyl ether pharmacophore with natural tetramic acids as lead compounds. New J Chem 45:5621. https://doi.org/10.1039/D1NJ00119A
Jiao J, Chen M, Sun S-X, Si W-J, Wang X-B, Ding W-J, Fu X-C, Wang A, Yang C-L (2021) Synthesis, bioactivity evaluation, 3D-QSAR, and molecular docking of novel pyrazole-4-carbohydrazides as potential fungicides targeting succinate dehydrogenase. Chin J Chem 39:323–329. https://doi.org/10.1002/cjoc.202000438
Wang X-B, Chai J-Q, Kong X-Y, Jin F, Chen M, Yang C-L (2021) Expedient discovery for novel antifungal leads: 1,3,4-oxadiazole derivatives bearing a quinazolin-4(3H)-one fragment. Bioorg Med Chem 45:116330. https://doi.org/10.1016/j.bmc.2021.116330
Mehan AS, Senturk E, Kara EM, Yusufoglu AS (2019) Synthesis, E/Z isomerization, and antimicrobial studies of different structured novel ketone derivatives. JOTCSA 6(2):177−188. https://doi.org/10.18596/jotcsa.486487
Jebli N, Arfaoui Y, Hecke KV, Cavalier JF, Touil S (2021) Experimental and computational investigation of Z/E isomerism, X-ray crystal structure and molecular docking study of (2-(hydroxyimino)cyclohexyl)diphenylphosphine sulfifide, a potential antibacterial agent. J Mol Struc 1229:129634. https://doi.org/10.1016/j.molstruc.2020.129634
Emami S, Falahati M, Banifatemi A, Moshirl K, Shafiee A (2002) Stereoselective synthesis and in vitro antifungal evaluation of (E)- and (Z)-imidazolylchromanone oxime ethers. Arch Pharm Med Chem 7:318−324. https://doi.org/10.1002/1521-4184(200209)335:7<318::AID-ARDP318>3.0.CO;2-O
Bolel P, Mahapatra N, Datta S, Halder M (2013) Modulation of accessibility of subdomain IB in the pH-dependent nteriaction of bovine serum albumin with cochineal red A: a combined view from spectroscopy and docking simulations. J Agric Food Chem 61:4606–4613. https://doi.org/10.1021/jf305395n
Jeger MJ, Lamour A, Gilligan CA, Otten W (2008) A fungal growth model fitted to carbon-limited dynamics of Rhizoctonia solani. New Phytologist 178: 625–633. https://doi.org/10.1111/j.1469-8137.2008.02394.x
Dyer RB, D.Plattner R, F.kendra D, Brown DW (2005) Fusarium graminearum TRI14 is required for high virulence and DON productionon wheat but not for DON synthesis in vitro. J Agric Food Chem 53:9281−9287. https://doi.org/10.1021/jf051441a
Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL (2017) Pathogen profile Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8(5):561–580. https://doi.org/10.1111/j.1364-3703.2007.00417.x
Diao X, Han Y-Y, Liu C-L (2018) The fungicidal activity of tebuconazole enantiomers against Fusarium graminearum and its selective effecton DON production under different conditions. J Agric Food Chem 66:3637–3643. https://doi.org/10.1021/acs.jafc.7b05483
Fan L-L, Luo Z-F, Yang C-F, Guo B, Miao J, Chen Y (2021) Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi. Mol Divers. https://doi.org/10.1007/s11030-021-10213-7
Liu S-M, Chen Y, Yu J-J, Chen C-J, Wang Z-X, Zhou M-G (2010) Transfer of the beta-tubulin gene of botrytis cinerea with resistance to carbendazim into Fusarium graminearum. Pest Manag Sci 66(5):482–489. https://doi.org/10.1002/ps.1897
Zhang Z-J, Zeng Y, jiang Z-Y, Shu B-S, Sethuraman V, Zhong G-H (2018) Design, synthesis, fungicidal property and QSAR studies of novel β-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rices heath blight. Pest Manag Sci 74:1736–1746. https://doi.org/10.1002/ps.4873
Yan X-J, Liang X-M, Jin S-H, Lv J-P, Yu C-X, Qin W-Y, Li B-J, Yuan H-J, Qi S-H, Shi Y-X, Wu J-P, Chen F-H, Wang D-Q (2010) Primary study on mode of action for macrocyclic fungicide candidates (7B3, D1) against Rhizoctonia solani Kuhn. J Agric Food Chem 58:2726–2729. https://doi.org/10.1021/jf9037369
Acknowledgements
The authors gratefully acknowledge the grants from the National Natural Science Foundation of China (No. 31772209) and the Fundamental Research Funds for the Central Universities of China (No. KYTZ201604).
Funding
Funding was provided by Innovative Research Group Project of the National Natural Science Foundation of China (Grant No. 31772209), Fundamental Research Funds for the Central Universities (Grant No. KYTZ201604).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A
Appendix A
The physical and spectroscopic data, crystal structure information and molecular docking related information of target compounds are provided in the Supporting Information. The copies of corresponding 1 H NMR, 13C NMR, 19F NMR, and HRMS spectrograms are also presented in the Supporting Information.
Rights and permissions
About this article
Cite this article
Sun, S., Yan, J., Tai, L. et al. Novel (Z)/(E)-1,2,4-triazole derivatives containing oxime ether moiety as potential ergosterol biosynthesis inhibitors: design, preparation, antifungal evaluation, and molecular docking. Mol Divers 27, 145–157 (2023). https://doi.org/10.1007/s11030-022-10412-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10412-w