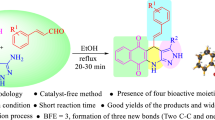
Abstract
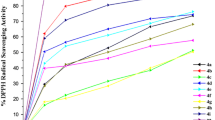
A one-pot synthesis of 3-alkoxycarbonyl-3,4-dihydro-2H-pyran-2-ones from intermolecular hetero-Diels–Alder reaction between vinylidene Melderum’s acids and dialkyl acetylenedicarboxylates, in the presence of simple alcohols at room temperature, is described. The advantages of this procedure are good yields, short reaction time, and easy workup. Antioxidant properties of four derivatives of these 3,4-dihydro-2H-pyran-2-ones, together with their antimicrobial activities, are investigated.
Graphical abstract






Similar content being viewed by others
References
Kalaria PN, Karad SC, Ravel DK (2018) A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur J Med Chem 158:917–936. https://doi.org/10.1016/j.ejmech.2018.08.040
Desai N, Trivedi A, Pandit U, Dodiya A, Rao VK, Desai P (2016) Hybrid bioactive heterocyclic as potential antimicrobial agents a review. Mini Rev Med Chem 16:1500–1526. https://doi.org/10.2174/1389557516666160609075620
Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, Fernandes AR (2015) Heterocyclic anticancer compounds recent advances and the paradigm shift towards the use of nano medicine’s tool box. Molecules 20:16852–16891. https://doi.org/10.3390/molecules200916852
Li W, Zhao SJ, Gao F, Lv ZS, Tu JY, Xu Z (2018) Synthesis and in vitro anti-tumor, anti-mycobacterial and anti-hiv activities of diethylene-glycol-tethered bis-isatin derivatives. ChemistrySelect 3:10250–10254. https://doi.org/10.1002/slct.201802185
Zhao X, Chaudhry ST, Mei J (2017) Heterocyclic building blocks for organic semiconductors. Adv Heterocycl Chem 121:133–171. https://doi.org/10.1016/bs.aihch.2016.04.009
Khattab TA, Rehan MA (2018) A review on synthesis of nitrogen-containing heterocyclic dyes for textile fibers-Part 1: five and six-membered heterocycles. Egypt J Chem 61:989–1018. https://doi.org/10.21608/ejchem.2018.4130.1362
Lamberth C, Dinges J (2012) Bioactive heterocyclic compound classes: agrochemicals. Wiley, ISBN: 978-3-527-33396-7
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu HR, Li Q (2012) Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac J Cancer Prev 13:5329–5343. https://doi.org/10.7314/apjcp.2012.13.11.5339
Tsuchiya K, Kobayashi S, Nishikiori T, Nakagawa T, Tatsuta K (1997) NK10958P, a novel plant growth regulator produced by Streptomyces sp. J Antibiot 50:259–260. https://doi.org/10.7164/antibiotics.50.259
Kondoh M, Usui T, Kobayashi S, Tsuchiya K, Nishikawa K, Nishikiori T, Mayumi T, Osada H (1998) Cell cycle arrest and antitumor activity of pironetin and its derivatives. Cancer Lett 126:29–32. https://doi.org/10.1016/s0304-3835(97)00528-4
Turner SR, Strohbach JW, Tommasi RA, Aristoff PA, Johnson PD, Shulnick HI, Dolak LA, Seest EP, Tomich PK, Bohanon MJ, Horng MM, Lynn JC, Chong KT, Hinshaw RR, Waterpaugh KD, Janakiraman MN, Thaisrivongs S (1998) Tipranavir (PNU-140690): a potent, orally bioavailable nonpeptidic HIV protease inhibitor of the 5,6-dihydro-4-hydroxy-2-pyrone sulfonamide class. J Med Chem 41:3467–3476. https://doi.org/10.1007/s00706-019-02388-5
Marty FM, Man CY, van der Horst C, Francois B, Garot D, Manez R, Thamlikitkul V, Lorente JA, Lerma FA, Brealey D, Zhao HH, Weller S, Yates PJ, Peppercorn AF (2014) Human monoclonal antibody 81.39a effectively neutralizes emerging influenza a viruses of group 1 and 2 hemagglutinins. J Infect Dis 209:542–550. https://doi.org/10.1128/JVI.01284-16
Omura S, Kuno F, Otoguro K, Sunazuka T, Shiomi K, Mamuma R, Iwai Y (1995) Arisugacin a novel and selective inhibitor of acetylcholinesterase from Penicillium sp. FO-4259. J Antibiot 48:745–746. https://doi.org/10.7164/antibiotics.48.745
Ozturk N, Korkmaz S, Ozturk Y, Baser KHC (2005) Effects of gentiopicroside sweroside and swertiamarine secoiridoi.orgds from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Med 72:289–294. https://doi.org/10.1055/s-2005-916198
Loepfe M, Linden A, Heimgartner H (2011) N-Methyl-N-phenyl-5-oxa-1-azaspiro[2.5]oct-1-en-2-amine-synthesis and reactions of a synthon for an unknown α-amino acid. Heterocycles 82:1267–1282. https://doi.org/10.5167/uzh-47460
Walter M, Von Coburg Y, Isensee K, Sander K, Stark H, Ligneau X, Camelin JC, Schwartz JC (2010) Azole derivatives as histamine H3 receptor antagonists, part I: thiazol-2-yl ethers. Bioorg Med Chem Lett 20:5879–5882. https://doi.org/10.1016/j.bmcl.2010.07.098
Jamieson C, Campbell RA, Cumming IA, Gillen KJ, Gillespie J, Kiczun M, Lamont Y, Lyons AJ, MacLean JKF, Moir EM, Morrow JA, Papakosta M, Rankovic Z, Smith L, Basten S, Kazemier B (2010) A novel series of positive modulators of the AMPA receptor: discovery and structure based hit-to-lead studies. Bioorg Med Chem Lett 20:5753–5756. https://doi.org/10.1016/j.bmcl.2010.07.138
Yavari I, Bayat M (2003) A new synthesis of highly functionalized 2H-pyran derivatives. Tetrahedron 59:2001–2005. https://doi.org/10.1016/S0040-4020(03)00114-5
Yavari I, Sirouspour M, Souri S (2006) Three-component synthesis of functionalized 5-oxo-4,5-dihydroindeno[1,2-b]pyrans. Mol Divers 10:265–270. https://doi.org/10.1007/s11030-006-9022-8
Azizi N, Ahooie TS, Hashemi MM, Yavari I (2018) Magnetic graphitic carbon nitride-catalyzed highly efficient construction of functionalized 4H-pyrans. Synlett 29:645–649. https://doi.org/10.1055/s-0036-1589145
Yavari I, Sheykhahmadi J (2021) TFA-mediated synthesis of functionalized pyrano[2,3-c]pyrazoles from pyrazol-3-ones, active carbonyl compounds and tert-BuOH. Mol Divers. https://doi.org/10.1007/s11030-021-10200-y
Heravi MM, Ahmadi T, Ghavidel M, Heidari B, Hamidi H (2015) Recent applications of the hetero Diels–Alder reaction in the total synthesis of natural products. RSC Adv 5:101999–102075. https://doi.org/10.1039/C5RA17488K
Bigi F, Carloni S, Ferrari L, Maggi R, Mazzacani A, Tarto GS (2001) Clean synthesis in water part 2: uncatalysed condensation reaction of meldrum’s acid and aldehydes. Tetrahedron Lett 42:5203–5205. https://doi.org/10.1016/S0040-4039(01)00978-9
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948. https://doi.org/10.1021/jf00018a005
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 42:629–632. https://doi.org/10.1021/jf00039a005
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49:4083–4089. https://doi.org/10.1021/jf0103572
Acknowledgements
We gratefully acknowledge from Science and Research Branch, Islamic Azad University, Tehran, Iran because of spiritual support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khandan, S., Yavari, I. & Azizian, J. A one-pot synthesis 3-alkoxycarbonyl-3,4-dihydro-2H-pyran-2-ones from vinylidene melderum’s acids, dialkyl acetylenedicarboxylates, and simple alcohols. Mol Divers 27, 125–133 (2023). https://doi.org/10.1007/s11030-022-10407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10407-7