Abstract
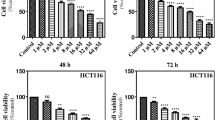
Colorectal cancer (CRC), especially metastatic (mCRC) form, becomes a major reason behind cancer morbidity worldwide, whereas the treatment strategy is not optimum. Several novel targets are under investigation for mCRC including the autophagy pathway. Natural compounds including dietary lignans are sparsely reported as autophagy modulators. Nonetheless, the interaction between dietary lignans and core autophagy complexes are yet to be characterised. We aimed to describe the interaction between the dietary lignans from flaxseed (Linum usitatissimum) and sesame seeds (Sesamum indicum) along with the enterolignans (enterodiol and enterolactone) and the UNC-51-like kinase 1 and 2 (ULK1/2), important kinases required for the autophagy. A range of in-silico technologies viz. molecular docking, drug likeness, and ADME/T was employed to select the best fit modulator and/or inhibitor of the target kinases from the list of selected lignans. Drug likeness and ADME/T studied further selected the best-suited lignans as potential autophagy inhibitor. Molecular dynamic simulation (MDS) analyses were used to validate the molecular docking results. Binding free energies of the protein–ligand interactions by MM-PBSA method further confirmed best-selected lignans as ULK1 and/or ULK2 inhibitor. In conclusion, three dietary lignans pinoresinol, medioresinol, and lariciresinol successfully identified as dual ULK1/2 inhibitor/modifier, whereas enterodiol emerged as a selective ULK2 inhibitor/modifier.
Graphical abstract








Similar content being viewed by others
Abbreviations
- CASTp:
-
Computed atlas of surface topography of proteins
- CRC:
-
Colorectal cancer
- mCRC:
-
Metastatic colorectal cancer
- MDS:
-
Molecular dynamics simulation
- MM-PBSA:
-
Molecular mechanics Poisson–Boltzmann surface area
- RMSD:
-
Root-mean-square deviation
- RMSF:
-
Root-mean-square fluctuations
- ULK:
-
Unc-51-like kinase
References
Perisetti A (2020) Aspirin for prevention of colorectal cancer in the elderly: friend or foe? Ann Gastroenterol. https://doi.org/10.20524/aog.2020.0556
Patel S, Hurez V, Nawrocki ST et al (2016) Vorinostat and hydroxychloroquine improve immunity and inhibit autophagy in metastatic colorectal cancer. Oncotarget 7:59087–59097. https://doi.org/10.18632/oncotarget.10824
McQuade RM, Stojanovska V, Bornstein JC, Nurgali K (2017) Colorectal cancer chemotherapy: the evolution of treatment and new approaches. CMC. https://doi.org/10.2174/0929867324666170111152436
Petrelli F, Perego G, Ghidini A et al (2020) A systematic review of salvage therapies in refractory metastatic colorectal cancer. Int J Colorectal Dis 35:783–794. https://doi.org/10.1007/s00384-020-03571-5
Taylor MA, Das BC, Ray SK (2018) Targeting autophagy for combating chemoresistance and radioresistance in glioblastoma. Apoptosis 23:563–575. https://doi.org/10.1007/s10495-018-1480-9
Panda PK, Mukhopadhyay S, Das DN et al (2015) Mechanism of autophagic regulation in carcinogenesis and cancer therapeutics. Semin Cell Dev Biol 39:43–55. https://doi.org/10.1016/j.semcdb.2015.02.013
Skarkova V, Kralova V, Vitovcova B, Rudolf E (2019) Selected aspects of chemoresistance mechanisms in colorectal carcinoma—a focus on epithelial-to-mesenchymal transition, autophagy, and apoptosis. Cells 8:234. https://doi.org/10.3390/cells8030234
Guan J-L, Simon AK, Prescott M et al (2013) Autophagy in stem cells. Autophagy 9:830–849. https://doi.org/10.4161/auto.24132
Long J, He Q, Yin Y et al (2020) The effect of miRNA and autophagy on colorectal cancer. Cell Prolif. https://doi.org/10.1111/cpr.12900
Zhang A, He W, Shi H et al (2016) Natural compound oblongifolin C inhibits autophagic flux, and induces apoptosis and mitochondrial dysfunction in human cholangiocarcinoma QBC939 cells. Mol Med Rep 14:3179–3183. https://doi.org/10.3892/mmr.2016.5591
Jia S, Xu X, Zhou S et al (2019) Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis 10:142. https://doi.org/10.1038/s41419-019-1366-y
Wang G, Wang T, Zhang Y et al (2019) Schizandrin protects against OGD/R-induced neuronal injury by suppressing autophagy: involvement of the AMPK/mTOR pathway. Molecules 24:3624. https://doi.org/10.3390/molecules24193624
Patra S, Mishra SR, Behera BP et al (2020) Autophagy-modulating phytochemicals in cancer therapeutics: current evidences and future perspectives. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.05.008
Rodríguez-García C, Sánchez-Quesada C, Toledo E et al (2019) Naturally lignan-rich foods: a dietary tool for health promotion? Molecules 24:917. https://doi.org/10.3390/molecules24050917
Landete JM (2012) Plant and mammalian lignans: a review of source, intake, metabolism, intestinal bacteria and health. Food Res Int 46:410–424. https://doi.org/10.1016/j.foodres.2011.12.023
Benvenuto M, Albonici L, Focaccetti C et al (2020) Polyphenol-mediated autophagy in cancer: evidence of in vitro and in vivo studies. IJMS 21:6635. https://doi.org/10.3390/ijms21186635
De Silva SF, Alcorn J (2019) Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 12:68. https://doi.org/10.3390/ph12020068
Eeckhaut E, Struijs K, Possemiers S et al (2008) Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J Agric Food Chem 56:4806–4812. https://doi.org/10.1021/jf800101s
Mali AV, Padhye SB, Anant S et al (2019) Anticancer and antimetastatic potential of enterolactone: clinical, preclinical and mechanistic perspectives. Eur J Pharmacol 852:107–124. https://doi.org/10.1016/j.ejphar.2019.02.022
Lampe JW, Kim E, Levy L et al (2019) Colonic mucosal and exfoliome transcriptomic profiling and fecal microbiome response to a flaxseed lignan extract intervention in humans. Am J Clin Nutr 110:377–390. https://doi.org/10.1093/ajcn/nqy325
Kaushik G, Ramalingam S, Subramaniam D et al (2012) Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. Am J Surg 204:868–873. https://doi.org/10.1016/j.amjsurg.2012.09.001
Lin C-J, Chen T-L, Tseng Y-Y et al (2016) Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl Pharmacol 304:59–69. https://doi.org/10.1016/j.taap.2016.05.018
Cheng Y-C, Hueng D-Y, Huang H-Y et al (2016) Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 7:29116–29130. https://doi.org/10.18632/oncotarget.8674
Corona Velazquez AF, Jackson WT (2018) So many roads: the multifaceted regulation of autophagy induction. Mol Cell Biol. https://doi.org/10.1128/MCB.00303-18
Banik K, Ranaware AM, Deshpande V et al (2019) Honokiol for cancer therapeutics: a traditional medicine that can modulate multiple oncogenic targets. Pharmacol Res 144:192–209. https://doi.org/10.1016/j.phrs.2019.04.004
Petherick KJ, Conway OJL, Mpamhanga C et al (2015) Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem 290:11376–11383. https://doi.org/10.1074/jbc.C114.627778
Sain A, Kandasamy T, Naskar D (2021) In silico approach to target PI3K/Akt/mTOR axis by selected Olea europaea phenols in PIK3CA mutant colorectal cancer. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1953603
Trott O, Olson AJ (2009) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem NA-NA. https://doi.org/10.1002/jcc.21334
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Pires DEV, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 58:4066–4072. https://doi.org/10.1021/acs.jmedchem.5b00104
Yang H, Lou C, Sun L et al (2019) admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 35:1067–1069. https://doi.org/10.1093/bioinformatics/bty707
Kandasamy T, Sudhamalla B, Naskar D (2020) Designing of RNA aptamer against DNA binding domain of the glucocorticoid receptor: a response element-based in-silico approach. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1822918
Schüttelkopf AW, van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr D Biol Crystallogr 60:1355–1363. https://doi.org/10.1107/S0907444904011679
ORCID. https://orcid.org/0000-0001-6661-8653. Accessed 25 Nov 2021
Kumari R, Kumar R, Lynn A (2014) Open source drug discovery consortium, g_mmpbsa —A GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54:1951–1962. https://doi.org/10.1021/ci500020m
Kollman PA, Massova I, Reyes C et al (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res 33:889–897. https://doi.org/10.1021/ar000033j
Dannenberg JJ (1998) An introduction to hydrogen bonding By George A. Jeffrey (University of Pittsburgh). Oxford University Press: New York and Oxford. 1997. ix + 303 pp. $60.00. ISBN 0–19–509549–9. J Am Chem Soc 120:5604–5604. Doi: https://doi.org/10.1021/ja9756331
Wang C, Greene D, Xiao L et al (2018) Recent developments and applications of the MMPBSA method. Front Mol Biosci 4:87. https://doi.org/10.3389/fmolb.2017.00087
Reif MM, Oostenbrink C (2014) Net charge changes in the calculation of relative ligand-binding free energies via classical atomistic molecular dynamics simulation. J Comput Chem 35:227–243. https://doi.org/10.1002/jcc.23490
Liu L, Yan L, Liao N et al (2020) A review of ULK1-mediated autophagy in drug resistance of cancer. Cancers 12:352. https://doi.org/10.3390/cancers12020352
Tian W, Chen C, Lei X et al (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 46:W363–W367. https://doi.org/10.1093/nar/gky473
Tan KP, Nguyen TB, Patel S et al (2013) Depth: a web server to compute depth, cavity sizes, detect potential small-molecule ligand-binding cavities and predict the pKa of ionizable residues in proteins. Nucleic Acids Res 41:W314–W321. https://doi.org/10.1093/nar/gkt503
Bommareddy A, Zhang XY, Kaushik RS, Dwivedi C (2010) Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: possible mechanisms of flaxseed on colon cancer development in animals. Drug Discov Ther 4:184–189
Zou Y, Chen Z, He X et al (2015) High expression levels of unc-51-like kinase 1 as a predictor of poor prognosis in colorectal cancer. Oncol Lett 10:1583–1588. https://doi.org/10.3892/ol.2015.3417
Acknowledgements
This work was supported by the Department of Science and Technology, Govt. of India (DST)—Inspire faculty research grant (DST/INSPIRE/04/2017/000675), and Maulana Abul Kalam Azad University of Technology, West Bengal (MAKAUT, WB) research seed grant to DN. It was further supported by the Council of Scientific and Industrial Research (CSIR), Govt. of India, in form of research fellowship to AS.
Author information
Authors and Affiliations
Contributions
Conceptualisation was performed by AS. Methodology, software, data curation, visualisation and investigation were performed by AS and TK. AS, TK and DN were responsible for writing original draft preparation and writing, reviewing and editing. DN performed supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sain, A., Kandasamy, T. & Naskar, D. Targeting UNC-51-like kinase 1 and 2 by lignans to modulate autophagy: possible implications in metastatic colorectal cancer. Mol Divers 27, 27–43 (2023). https://doi.org/10.1007/s11030-022-10399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10399-4