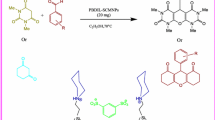
Abstract
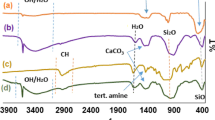
In this research, the synthesis of novel indeno[1,2-b]pyrano[2,3-f]chromene-2,12(13H)-dione derivatives in the presence of a newly introduced magnetically recoverable nanosolid acid catalyst is reported. At the first, phosphoric acid-functionalized silica-coated Fe3O4 nanoparticles (Fe3O4@SiO2–(CH2)3OPO3H2) were prepared and well characterized using infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), vibrating sample magnetometer (VSM), and energy-dispersive X-ray spectroscopy (EDS) techniques. Then, the catalytic activity of the prepared Fe3O4@ SiO2–(CH2)3OPO3H2 nanocatalyst was investigated for the synthesis of novel indeno[1,2-b]pyrano[2,3-f]chromene-2,12(13H)-dione derivatives via a one-pot and three-component condensation between 5,7-dihydroxy-4-methylcoumarin, indane-1, 3-dione, and various aromatic aldehydes under solvent-free condition. All the products are unknown, and their characterization was performed with the spectral data information obtained from their FT-IR, 1H and 13CNMR, elemental analysis, and their melting points. The reusability study of the introduced nanosolid acid catalyst showed that the catalytic stability is almost completely remained up to five consecutive runs.
Graphical Abstract










Similar content being viewed by others
References
Motokura K, Ding S, Usui K, Kong Y (2018) ACS Catal 11(2021):11985–11991
Gill CG, Price BA, Jones WJ (2007) J Catal 251:145–152
Lin Y, Chen H, Lin K, Chen B, Chiou C (2011) J Environ Sci 23:44–50
Mahmoodi NM, Khorramfar S, Najafi F (2011) Desalination 279:61–68
Kiasat AR, Nazari S (2012) J Mol Catal A: Chem 365:80–86
Khoobi M, Mamani L, Rezazadeh F, Zareie Z, Foroumadi A, Ramazani AR, Shafiee A (2012) J Mol Catal A: Chem 359:74–80
Costa VV, Jacinto MJ, Rossi LM, Landers R, Gusevskaya EV (2011) J Catal 282:209–214
Lu AH, Salabas EL, Schüth F (2007) Angew Chem Int Ed 46:1222–1244
Xu Z, Pupek K, Suling W, Enache L, Flavin M (2006) Bioorg Med Chem 14:4610
Wu J, Fong W, Zhang J, Leung C, Kwong H, Yang M, Li D, Cheung H (2003) Eur J Pharmacol 473:9
Sethna SM, Shah NM (1945) Chem Rev 36:1
Neyts J, Clercq ED, Singha R, Chang YH, Das AR, Chakraborty SK, Hong SC, Hsu MH, Hwu JR (2009) J Med Chem 52:1486
Hwu JR, Singha R, Hong SC, Chang YH, Das AR, Vliegen I, Clercq ED, Neyts J (2008) Antiviral Res 77:157
Sharma RC, Parashar RK (1988) J Inorg Biochem 32:163
Kayser O, Kolodziej HZ, Naturforsch ZC (1999) Biosci 54:169
Garazd YL, Kornienko EM, Maloshtan LN, Garazd MM, Khilya VP (2005) Chem Nat Prod 41:508
Kontogiorgis CA, Hadjipavlou-Litina DJ (2005) J Med Chem 48:6400
Suzuki M, Nakagawa-Goto K, Nakamura S, Tokuda H, Morris-Natschke SL, Kozuka M, Nishino H, Lee KH (2006) Pharm Biol 44:178
Parker KA, Mindt TL (2011) Tetrahedron 67:9779
Buchlovic M, Man S, Kislitson K, Mathot C, Potacek M (2010) Tetrahedron 66:1821
Zarganes-Tzitzikas T, Terzidis MA, Stephanidou-Stephanatou J, Tsoleridis CA, Kostakis GE (2011) J Org Chem 76:9008
Taib LA, Keshavarz M (2021) Res Chem Intermed 47:2487–2505
Karami B, Mohammadpour-Dehghani F, Eskandari K (2012) Croat Chem Acta 85:147
Keshavarz M, Zarei Ahmady A, Vaccaro L, Kardani M (2018) Molecules 23(2018):330–346
Keshavarz M, Tabatabaee M, Shahabi M, Yazdankish E (2021) Polycycl Aromat Compd 41:427–439
Keshavarz M (2016) J Iran Chem Soc 13:553–561
Keshavarz M, Iravani N, Ahmadi Azqhandi MH, Nazari S (2016) Res Chem Intermed 42:4591–4604
Taib LA, Keshavarz M, Parhami A (2021) React Kinet Mech Catal 133:383–403
Karami B, Farahi M, Farmani N, MohamadiTanuraghaj H (2016) New J Chem 40:1715–1719
Khosravian F, Karami B, Farahi M (2017) New J Chem 41:11584–11590
Farahi M, Karami B, Keshavarz R, Khosravian F (2017) RSC Adv 7:46644–46650
Arian F, Keshavarz M, Sanaeishoar H, Hasanzadeh N (2021) J Mol Struct 1229:129599
Rohani L, Karami B, Farahi M (2021) J Chin Chem Soc 68:888–892
Akrami S, Karami B, Farahi M (2020) J Heterocycl Chem 57:2446–2454
Karami B, Khodabakhshi S, Eskandari K (2012) Tetrahedron Lett 53:1445–1446
Mascolo MC, Pei Y, Ring TA (2013) Materials 6:5549
Stober W, Fink A, Bohn E (1968) J Colloid Interface Sci 26:62
Elmekawy AA, Sweeney JB, Brown DR (2015) Catal Sci Technol 5:690–696
Nasseri MA, Zakerinasab B, Samieadel MM (2014) RSC Adv 4:41753–41762
Yang P, Quan Z, Li C, Kang X, Lian H, Lin J (2008) Biomaterials 29:4341–4347
Gupta K, Agarwal S, Saleh TA (2011) Water Res 45:2207–2212
Sharma S, Gupta A, Chik SMST, Kee CG, Mistry BM, Kim DH, Sharma G (2017) Int J Biol Macromol 104:189–196
Acknowledgements
The authors gratefully acknowledge the partial support of this work by Yasouj University, Yasouj, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sedighimehr, I., Karami, B., Farahi, M. et al. Synthesis of novel pyrano[2,3-f]chromene-dione derivatives using phosphoric acid-functionalized silica-coated Fe3O4 nanoparticles as a new reusable solid acid nanocatalyst. Mol Divers 26, 3325–3336 (2022). https://doi.org/10.1007/s11030-022-10393-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10393-w