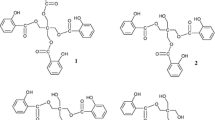
Abstract
Selected salicylidene imines were evaluated for their antioxidant and cytotoxic potentials. Several of them exerted potent scavenging capacity towards ABTS radical and hydrogen peroxide. The insight into the preferable antioxidative mechanism was reached employing density functional theory. In the absence of free radicals, the SPLET mechanism is dominant in polar surroundings, while HAT is prevailing in a non-polar environment. The results obtained for the reactions of the most active compounds with some medically relevant radicals pointed out competition between HAT and SPLET mechanisms. The assessment of their cytotoxic properties revealed inhibition of ER-a human breast adenocarcinoma cells or estrogen-independent prostate cancer cells. Molecular docking study with the cyclooxygenase (COX) 2 enzyme was performed to examine the most probable bioactive conformations and possible interactions between the tested derivatives and COX-2 binding pocket.
Graphical abstract



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Hodnett E, Dunn W (1970) Structure-antitumor activity correlation of some Schiff bases. J Med Chem 13:768–770. https://doi.org/10.1021/jm00298a054
Da Silva C et al (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2:1–8. https://doi.org/10.1016/j.jare.2010.05.004
Sztanke K et al (2013) An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg Med Chem 21:3648–3666. https://doi.org/10.1016/j.bmc.2013.04.037
Ariyaeifar M et al (2018) Chiral halogenated Schiff base compounds: green synthesis, anticancer activity and DNA-binding study. J Mol Struct 1161:497–511. https://doi.org/10.1016/j.molstruc.2018.02.042
Bharathi Dileepan A et al (2018) Isatin based macrocyclic Schiff base ligands as novel candidates for antimicrobial and antioxidant drug design: In vitro DNA binding and biological studies. J Photochem Photobiol B Biol 183:191–200. https://doi.org/10.1016/j.jphotobiol.2018.04.029
García-Valle F et al (2018) Aluminates with fluorinated Schiff bases: influence of the alkali metal-fluorine interactions in structure stabilization. Molecules 23:3108. https://doi.org/10.3390/molecules23123108
Tan X et al (2019) Synthesis, structure and antiproliferative and optical activities of two new biphenyl-derived Schiff bases. Acta Cryst C 75:97–106. https://doi.org/10.1107/S2053229618017989
Shao J et al (2005) Determination of the potency and subunit-selectivity of ribonucleotide reductase inhibitors with a recombinant-holoenzyme-based in vitro assay. Biochem Pharmacol 69:627–634. https://doi.org/10.1016/j.bcp.2004.11.016
Gama S et al (2011) Copper(II) complexes with tridentate pyrazole-based ligands: synthesis, characterization, DNA cleavage activity and cytotoxicity. J Inorg Biochem 105:637–644. https://doi.org/10.1016/j.jinorgbio.2011.01.013
Kraicheva I et al (2012) Synthesis, antiproliferative activity and genotoxicity of novel anthracene-containing aminophosphonates and a new anthracene-derived Schiff base. Bioorg Med Chem 20:117–124. https://doi.org/10.1016/j.bmc.2011.11.024
Kamel M et al (2010) Synthesis, antitumor activity and molecular docking study of novel Sulfonamide-Schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur J Med Chem 45:572–580. https://doi.org/10.1016/j.ejmech.2009.10.044
Vicini P et al (2003) Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 11:4785–4789. https://doi.org/10.1016/S0968-0896(03)00493-0
Tang Y, Liu Z (2007) Insight into the free-radical-scavenging mechanism of hydroxyl-substituent Schiff bases in the free-radical-induced hemolysis of erythrocytes. Cell Biochem Funct 25:701–710. https://doi.org/10.1002/cbf.1378
Zhang Y et al (2013) Synthesis and antioxidant activities of 2-oxo-quinoline-3-carbaldehyde Schiff-base derivatives. Bioorg Med Chem Lett 23:107–111. https://doi.org/10.1016/j.bmcl.2012.11.006
Kotora P et al (2016) The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 21:127. https://doi.org/10.3390/molecules21010127
Lushchak V (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5:1–8. https://doi.org/10.1186/1477-3163-5-14
Labunskyy V, Gladyshev V (2013) Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal 19:1362–1372. https://doi.org/10.1089/ars.2012.4891
Zhao F, Liu Z (2009) The protective effect of hydroxyl-substituted Schiff bases on the radical-induced oxidation of DNA. J Phys Org Chem 22:791–798. https://doi.org/10.1002/poc.1517
Yusuff O et al (2019) Kinetics and mechanism of the antioxidant activities of C. olitorius and V. amygdalina by Spectrophotometric and DFT methods. ACS Omega 4:13671–13680. https://doi.org/10.1021/acsomega.9b00851
Craft B et al (2012) Phenol-based antioxidants and the In Vitro methods used for their assessment. Compr Rev Food Sci Food Saf 11:148–173. https://doi.org/10.1111/j.1541-4337.2011.00173.x
Di Meo F et al (2013) Free radical scavenging by natural polyphenols: atom versus electron transfer. J Phys Chem 117:2082–2092. https://doi.org/10.1021/jp3116319
Farrokhnia M (2020) Density functional theory studies on the antioxidant mechanism and electronic properties of some bioactive marine meroterpenoids: Sargahydroquionic acid and sargachromanol. ACS Omega 5:20382–20390. https://doi.org/10.1021/acsomega.0c02354
Alov P, Tsakovska I, Pajeva I (2014) Computational studies of free radical-scavenging properties of phenolic compounds. Curr Top Med Chem 15:85–104. https://doi.org/10.2174/1568026615666141209143702
Kirschenbaum A et al (2001) The role of cyclooxygenase-2 in prostate cancer. Urology 58:127–131. https://doi.org/10.1016/S0090-4295(01)01255-9
Half E et al (2002) Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res 62:1676–1681
Singh-Ranger G, Mokbel K (2002) The role of cyclooxygenase-2 (COX-2) in breast cancer, and implications of COX-2 inhibition. Eur J Surg Oncol 28:729–737. https://doi.org/10.1053/ejso.2002.1329
Sooriakumaran P, Kaba R (2005) The risks and benefits of cyclo-oxygenase-2 inhibitors in prostate cancer: a review. Int J Surg 3:278–285. https://doi.org/10.1016/j.ijsu.2005.10.002
Mazhar D, Ang R, Waxman J (2006) COX inhibitors and breast cancer. Br J Cancer 94:346–350. https://doi.org/10.1038/sj.bjc.6602942
Khor L et al (2007) COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92–02 trial. Lancet Oncol 8:912–920. https://doi.org/10.1016/S1470-2045(07)70280-2
Singh B et al (2007) COX-2 involvement in breast cancer metastasis to bone. Oncogene 26:3789–3796. https://doi.org/10.1038/sj.onc.1210154
Csepregi K et al (2016) Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 21:1–17. https://doi.org/10.3390/molecules21020208
Velena A et al (2016) 1,4-dihydropyridine derivatives: dihydronicotinamide analogues-model compounds targeting oxidative stress. Oxid Med Cell Longev 2016:1892412. https://doi.org/10.1155/2016/1892412
Nikokavoura A et al (2011) Evaluation of antioxidant activity of hydrophilic and lipophilic compounds in edible oils by a novel fluorimetric method. Talanta 84:874–880. https://doi.org/10.1016/j.talanta.2011.02.007
Li S, Wang X, Kong L (2014) Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur J Med Chem 71:36–45. https://doi.org/10.1016/j.ejmech.2013.10.068
Slassi S et al (2019) Imidazole and Azo-based Schiff bases ligands as highly active antifungal and antioxidant components. Heteroat Chem 2019:1–8. https://doi.org/10.1155/2019/6862170
Yang H et al (2017) Design, synthesis and evaluation of coumarin-pargyline hybrids as novel dual inhibitors of monoamine oxidases and amyloid-β aggregation for the treatment of Alzheimer’s disease. Eur J Med Chem 138:715–728. https://doi.org/10.1016/j.ejmech.2017.07.008
Krokidis M et al (2019) Assessment of dna topoisomerase i unwinding activity, radical scavenging capacity, and inhibition of breast cancer cell viability of N-alkylacridones and N, Nʹ-dialkyl-9,9ʹ-biacridylidenes. Biomolecules 9:1–15. https://doi.org/10.3390/biom9050177
da Silva C et al (2017) Studies on free radical scavenging, cancer cell antiproliferation, and calf thymus DNA interaction of Schiff bases. J Photochem Photobiol B 172:129–138. https://doi.org/10.1016/j.jphotobiol.2017.05.020
Adsule S et al (2006) Novel Schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J Med Chem 49:7242–7246. https://doi.org/10.1021/jm060712l
Antonczak S (2008) Electronic description of four flavonoids revisited by DFT method. J Mol Struct-Theochem 856:38–45. https://doi.org/10.1016/j.theochem.2008.01.014
Petrović Z et al (2015) Experimental and theoretical study of antioxidative properties of some salicylaldehyde and vanillic Schiff bases. RSC Adv 5:24094–24100. https://doi.org/10.1039/c5ra02134k
Xie J, Schaich K (2014) Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J Agric Food Chem 62:4251–4260. https://doi.org/10.1021/jf500180u
Hammamieh R, Jett M (2008) Potential roles for inhibitors of arachidonic acid metabolism in prevention and treatment of breast cancer. Future Lipidol 3:265–271. https://doi.org/10.2217/17460875.3.3.265
Yang P et al (2012) Arachidonic acid metabolism in human prostate cancer. Int J Oncol 41:1495–1503. https://doi.org/10.3892/ijo.2012.1588
Pang L, Hurst E, Argyle D (2016) Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int 2016:2048731. https://doi.org/10.1155/2016/2048731
Hashemi Goradel N et al (2019) Cyclooxygenase-2 in cancer: a review. J Cell Physiol 234:5683–5699. https://doi.org/10.1002/jcp.27411
Brizzolara A et al (2017) The ErbB family and androgen receptor signaling are targets of Celecoxib in prostate cancer. Cancer Lett 400:9–17. https://doi.org/10.1016/j.canlet.2017.04.025
Shi L et al (2018) Celecoxib-induced self-assembly of smart albumin-doxorubicin conjugate for enhanced cancer therapy. ACS Appl Mater Interfaces 10:8555–8565. https://doi.org/10.1021/acsami.8b00875
Moustakali-Mavridis I, Hadjoudis E, Mavridis A (1978) Crystal and molecular structure of some thermochromic Schiff bases. Acta Crystallogr Sect B 34:3709–3715. https://doi.org/10.1107/S0567740878011930
Talati J, Desai M, Shah N (2005) Meta-substituted aniline-N-salicylidenes as corrosion inhibitors of zinc in sulphuric acid. Mater Chem Phys 93:54–64. https://doi.org/10.1016/j.matchemphys.2005.02.004
Chatziefthimiou S et al (2006) Keto forms of salicylaldehyde Schiff bases: Structural and theoretical aspects. J Phys Chem B 110:23701–23709. https://doi.org/10.1021/jp064110p
Koar B et al (2009) (E)-2-[(4-chloro-phen-yl)imino-meth-yl]-5-methoxy-phenol and (E)-2-[(2-chloro-phen-yl)imino-meth-yl]-5-methoxy-phenol: X-ray and DFT-calculated structures. Acta Crystallogr Sect C Cryst Struct Commun 65:8–13. https://doi.org/10.1107/S0108270109034350
Al-Kahraman Y et al (2010) Antileishmanial, antimicrobial and antifungal activities of some new aryl azomethines. Molecules 15:660–671. https://doi.org/10.3390/molecules15020660
Christodouleas D, Papadopoulos K, Calokerinos A (2011) Determination of total antioxidant activity of edible oils as well as their aqueous and organic extracts by chemiluminescence. Food Anal Methods 4:475–484. https://doi.org/10.1007/s12161-010-9189-6
Dl Christodouleas et al (2015) Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal Methods 8:1294–1302. https://doi.org/10.1007/s12161-014-0005-6
Frisch M et al (2016) G16_C01. p. Gaussian 16, Revision C.01, Gaussian, Inc., Wallin
Becke A (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Marenich A, Cramer C, Truhlar D (2009) Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J Phys Chem B 113:4538–4543. https://doi.org/10.1021/jp809094y
Marković Z et al (2016) Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput Theor Chem 1077:11–17. https://doi.org/10.1016/j.comptc.2015.09.007
Orlando B, Malkowski M (2016) Substrate-selective inhibition of cyclooxygeanse-2 by fenamic acid derivatives is dependent on peroxide tone. J Biol Chem 291:15069–15081. https://doi.org/10.1074/jbc.M116.725713
Pettersen E et al (2004) UCSF chimera-a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Simijonović D et al (2018) Dicoumarol derivatives: green synthesis and molecular modelling studies of their anti-LOX activity. Bioorg Chem 80:741–752. https://doi.org/10.1016/j.bioorg.2018.07.021
Fiser A, Šali A (2003) MODELLER: generation and refinement of homology-based protein structure models. Meth Enzymol 374:461–491. https://doi.org/10.1016/S0076-6879(03)74020-8
Kouzi O, Pontiki E, Hadjipavlou-Litina D (2019) 2-arylidene-1-indandiones as pleiotropic agents with antioxidant and inhibitory enzymes activities. Molecules 24:1–20. https://doi.org/10.3390/molecules24234411
Saeed A, Mumtaz A, Flörke U (2007) 1-(3,4-Dimethoxy-benz-oyl)-3,5-dimethyl-1H-pyrazole. Acta Crystallogr E 63(10):1098. https://doi.org/10.1107/S1600536807044960
Acknowledgements
This work was supported by the Serbian Ministry of Education, Science and Technological Development (Agreement No. 451-03-9/2021-14/200122).
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Branković, J., Krokidis, M.G., Dousi, I. et al. Antioxidant and cytotoxic activities of selected salicylidene imines: experimental and computational study. Mol Divers 26, 3115–3128 (2022). https://doi.org/10.1007/s11030-021-10370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10370-9