Abstract
Starting from the 3,5-dimethyl pyrazole ring and acetophenone derivatives, five different N-propargylated C-3 substituted pyrazoles were obtained. These derivatives were reacted with different amine derivatives using Cs2CO3 in methanol and 11 different pyrazolo [1,5-a] pyrazine-4(5H)-one derivatives were obtained, which are not found in the literature. The cytotoxic effects of these derivatives in the A549 cell line were investigated. The 160 µM concentration of two derivatives was found to increase cell death rate to 50%, and two derivatives increased cell death rate by up to 40%. The structure–activity relationship (SAR) study revealed an amide group with a long alkyl chain and benzene ring with a p-CF3 group could be important for efficiency. With theoretical ADMET studies of pyrazolopyrazine derivatives, pharmacokinetic phases were predicted to be suitable.
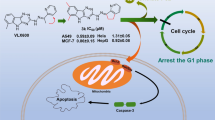
Graphic abstract






Similar content being viewed by others
References
Silvestri R, Cascio MG, Regina GL, Piscitelli F, Lavecchia A, Brizzi A, Pasquini S, Botta M, Novellino E, Marzo VD, Corelli F (2008) Synthesis, cannabinoid receptor affinity, and molecular modeling studies of substituted 1-Aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J Med Chem 51:1560. https://doi.org/10.1021/jm070566z
Szabó G, Fischer J, Kis-Varga Á, Gyires K (2008) New celecoxib derivatives as anti-ınflammatory agents. J Med Chem 51:142. https://doi.org/10.1021/jm070821f
Graneto MJ, Kurumbail RG, Vazquez ML, Shieh HS, Pawlitz JL, Williams JM, Stallings WC, Geng L, Naraian AS, Koszyk FJ, Stealey MA, Xu SD, Weier RM, Hanson GJ, Mourey RJ, Compton RP, Mnich SJ, Anderson JD, Monahan JB, Devraj R (2007) Synthesis, crystal structure, and activity of pyrazole-based ınhibitors of p38 kinase. J Med Chem 50:5712. https://doi.org/10.1021/jm0611915
Bildirici İ, Cetin A, Menges N, Alan Y (2018) Synthesis and SAR studies of pyrazole-3-carboxamides and -3-carbonyl thioureides including chiral moiety: novel candidates as antibacterial agents. J Serb Chem Soc 83:795. https://doi.org/10.2298/JSC170313029B
Langford HM, Williams PD, Homnick CF, Vacca JP, Felock PJ, Stillmock KA, Witmer MV, Hazuda DJ, Gabryelski LJ, Schleif WA (2008) Design and synthesis of substituted 4-oxo-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazine-2-carboxamides, novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett 18:721. https://doi.org/10.1016/j.bmcl.2007.11.049
Weber AE, Ashton WT (2006) Fused triazole derivatives as dipeptidyl peptidase-iv inhibitors for the treatment or prevention of diabetes, Pat. WO Appl. 2006023750
Zhang JH, Fan CD, Zhao BX, Shin DS, Dong WL, Xie YS, Miao JY (2011) Synthesis of novel pyrazolo[1,5-a]pyrazin-4(5H)-one derivatives and their inhibition against growth of A549 and H322 lung cancer cells. Bioorg Med Chem Lett 21:3909. https://doi.org/10.1016/j.bmcl.2011.05.035
Menges N, Sari O, Abdullayev Y, Erdem SS, Balci M (2013) Design and synthesis of pyrrolotriazepine derivatives: an experimental and computational study. J Org Chem 78:5184. https://doi.org/10.1021/jo4001228
Kuzu B, Genç H, Taşpinar M, Tan M, Menges N (2018) An easy synthetic protocol for imidazo-1,4-oxazines and evaluation of their toxicities. Heteroatom Chem 29:e21412. https://doi.org/10.1002/hc.21412
Çetinkaya Y, Balci M (2014) Selective synthesis of N-substituted pyrrolo[1,2-a]pyrazin-1(2H)-one derivatives via alkyne cyclization. Tetrahedron Lett 55:6698. https://doi.org/10.1016/j.tetlet.2014.10.044
Sari O, Seybek AF, Kaya S, Menges N, Erdem SS, Balci M (2019) Mechanistic ınsights into the reaction of N-propargylated pyrrole- and ındole-carbaldehyde with ammonia, alkyl amines, and branched amines: a synthetic and theoretical ınvestigation. Eur J Org Chem. https://doi.org/10.1002/ejoc.201900084
Surase YB, Samby K, Amale SR, Sood R, Purnapatre KP, Pareek PK, Das B, Nanda K, Kumar S, Verma AK (2017) Identification and synthesis of novel inhibitors of mycobacterium ATP synthase. Bioorg Med Chem Lett 27:3454. https://doi.org/10.1016/j.bmcl.2017.05.081
For the toxicity of triphenylphosphine, see the relevant datasheet. https://www.nwmissouri.edu/naturalsciences/sds/t/Triphenylphosphine.pdf
Snelders DJM, Yan N, Gan W, Laurenczy G, Dyson PJ (2012) Tuning the chemoselectivity of Rh nanoparticle catalysts by site-selective poisoning with phosphine ligands: the hydrogenation of functionalized aromatic compounds. ACS Catal 2:201. https://doi.org/10.1021/cs200575r
The American Cancer Society Medical and Editorial Content Team (2019) What is lung cancer? American Cancer Society. https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed 15 Dec 2020
Bethesda MD (2020) Non-small cell lung cancer treatment. Adult Treatment Editorial Board-National Cancer Institute. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Accessed 15 Dec 2020
Ricciuti B, Leonardi GC, Metro G, Grignani F, Paglialunga L, Bellezza G, Baglivo S, Mencaroni C, Baldi A, Zicari D, Crino L (2016) Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Review Res. Med. 10:53. https://doi.org/10.1586/17476348.2016.1115349
Pires DEV, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 58:4066–4072. https://doi.org/10.1021/acs.jmedchem.5b00104
Kharaneko AO (2016) Strategy for the synthesis of pyrazolo[5,1-d][1,2,5]triazepinones, a new heterocyclic system. Russ J Org Chem 52:1322. https://doi.org/10.1134/S1070428016090128
Hartner FW, Cvetovich RJ, Tsay FR, Amato JS, Pipik B, Grabowski EJ, Reider PJ (1999) A highly convergent synthesis of a fibrinogen receptor antagonist. J Org Chem 64:7751. https://doi.org/10.1021/jo990644t
Nasr T, Bondock S, Youns M, Fayad W, Zaghary W (2017) Synthesis, antitumor evaluation and microarray study of some new pyrazolo[3,4-d][1,2,3]triazine derivatives. Eur J Med Chem 141:603–614. https://doi.org/10.1016/j.ejmech.2017.10.016
Rikimaru K, Wakabayashi T, Abe H, Imoto H, Maekawa T, Ujikawa O, Murase K, Matsuo T, Matsumoto M, Nomura C, Tsuge H (2012) A new class of non-thiazolidinedione, non-carboxylic-acid-based highly selective peroxisome proliferator-activated receptor (PPAR) γ agonists: design and synthesis of benzylpyrazole acylsulfonamides. Bioorg Med Chem 20:714–733. https://doi.org/10.1016/j.bmc.2011.12.008
Acknowledgements
Authors thank The Scientific And Technological Research Council Of Turkey (TÜBİTAK- 119Z011) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uygun, M.T., Amudi, K., Turaçlı, İ.D. et al. A new synthetic approach for pyrazolo[1,5-a]pyrazine-4(5H)-one derivatives and their antiproliferative effects on lung adenocarcinoma cell line. Mol Divers 26, 113–124 (2022). https://doi.org/10.1007/s11030-020-10161-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10161-8