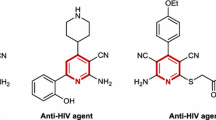
Abstract
2-Naphthol or β-naphthol is an important starting material that has drawn great attention in various organic transformations because of its attributes, such as low cost, easy to handle and eco-friendliness. The electron-rich aromatic framework of 2-naphthol with multiple reactive sites allows it to be utilized in several kinds of organic reactions eventuated to several organic molecules with potent biological properties. Multicomponent reaction approach has been tremendously utilized to explore the synthetic utility of 2-naphthol for the construction of diverse N/O-containing heterocyclic framework. In this review, we summarize recent data pertaining to multicomponent reactions, wherein heterocyclic compounds are synthesized utilizing 2-naphthol as one of the starting materials. It is anticipated that this review will stimulate the researchers to design new multicomponent strategies complying with the Green Chemistry principles for the further exploitation of 2-naphthol for the rapid synthesis of versatile biologically relevant heterocycles.
Graphic abstract
This review provides a concise overview of the different 2-naphthol based multicomponent reactions utilized for the construction of diverse bioactive heterocyclic scaffold.





















































Similar content being viewed by others
References
Kalaria PN, Karad SC, Raval DK (2018) A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur J Med Chem 158:917–936
Desai N, Trivedi A, Pandit U, Dodiya A, Rao VK, Desai P (2016) Hybrid bioactive heterocycles as potential antimicrobial agents: a review. Mini Rev Med Chem 16:1500–1526
Fouad MM, El-Bendary ER, Suddek GM, Shehata IA, El-Kerdawy MM (2018) Synthesis and in vitro antitumor evaluation of some new thiophenes and thieno[2,3-d]pyrimidine derivatives. Bioorg Chem 81:587–598
Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, Fernandes AR (2015) Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 20:16852–16891
Siddiqui N, Andalip Bawa S, Ali R, Afzal O, Akhtar MJ, Azad B, Kumar R (2011) Antidepressant potential of nitrogen-containing heterocyclic moieties: an updated review. J Pharm Bioallied Sci 3:194–212
Sokolova AS, Yarovaya OI, Bormotov NI, Shishkina LN, Salakhutdinov NF (2018) Synthesis and antiviral activity of camphor-based 1,3-thiazolidin 4-one and thiazole derivatives as Orthopoxvirus-reproduction inhibitors. MedChemComm 9:1746–1753
Goel A, Agarwal N, Singh FV, Sharon A, Tiwari P, Dixit M, Pratap R, Srivastava AK, Maulik PR, Ram VJ (2004) Antihyperglycemic activity of 2-methyl-3,4,5-triaryl-1H-pyrroles in SLM and STZ models. Bioorg Med Chem Lett 14:1089–1092
Amir M, Javed SA, Kumar H (2007) Pyrimidine as anti-inflammatory agent: a review. Indian J Pharm Sci 69:337–343
Li W, Zhao SJ, Gao F, Lv ZS, Tu JY, Xu Z (2018) Synthesis and in vitro anti-tumor, anti-mycobacterial and anti-HIV activities of diethylene-glycol-tethered bis-isatin derivatives. ChemistrySelect 3:10250–10254
Zhao X, Chaudhry ST, Mei J (2017) Heterocyclic building blocks for organic semiconductors. Heterocyclic chemistry in the 21st Century—a Tribute to Alan Katritzky 121:133–171
Khattab TA, Rehan MA (2018) Review on synthesis of nitrogen-containing heterocyclic dyes for textile fibers—part 2: fused heterocycles. Egypt J Chem 61:989–1018
Lamberth C, Dinges J (2012) Bioactive heterocyclic compound classes: agrochemicals. Wiley-VCH Verlag GmbH & Co, KGaA
Zhi S, Ma X, Zhang W (2019) Consecutive multicomponent reactions for the synthesis of complex molecules. Org Biomol Chem 17:7632–7650
Ibarra IA, Islas-Jácome A, González-Zamora E (2018) Synthesis of polyheterocycles via multicomponent reactions. Org Biomol Chem 16:1402–1418
Tietze LF, Bsasche C, Gericke KM (2006) Domino reactions in organic synthesis. Wiley-VCH, Weinheim
Weber L, Illgen M, Almstetter M (1999) Discovery of new multi component reactions with combinatorial methods. Synlett 3:366–374
Herrera RP, Marqués-López E (2015) Multicomponent reactions: concepts and applications for design and synthesis. Wiley, Hoboken
Naya A, Sagara Y, Ohwaki K, Saeki T, Ichikawa D, Iwasawa Y, Noguchi K, Ohtake N (2001) Design, synthesis, and discovery of a novel CCR1 antagonist. J Med Chem 44:1429–1435
Limsuwan S, Trip EN, Kouwen TR, Piersma S, Hiranrat A, Mahabusarakam W, Voravuthikunchai SP, Dijl JMV, Kayser O (2009) Rhodomyrtone: a new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 1:645–651
Carr AA, Grunwell JF, Sill AD, Meyer DR, Sweet FW, Scheve BJ, Grisar JM, Fleming RW, Mayer GD (1976) Bis-basic-substituted polycyclic aromatic compounds. A new class of antiviral agents. 7. Bisalkamine esters of 9-oxoxanthene-2,7-dicarboxylic acid, 3,6-bis-basic ethers of xanthen-9-one, and 2,7-bis(aminoacyl)xanthen-9-ones, -xanthenes, and –thioxanthenes. J Med Chem 19:1142–1148
Nishiyama T, Sakita K, Fuchigami T, Tsutomu Fukui T (1998) Antioxidant activities of fused heterocyclic compounds, xanthene-2,7-diols with BHT or catechol skeleton. Polym Degrad Stab 62:529–534
Zelefack F, Guilet D, Fabre N, Bayet C, Chevalle S, Ngouela S, Lenta BN, Valentin A, Tsamo E, Dijoux-Franca MG (2009) Cytotoxic and antiplasmodial xanthones from pentadesma butyracea. J Nat Prod 72:954–957
Banerjee A, Mukherjee AK (1981) Chemical aspects of santalin as a histological stain. Stain Technol 56:83–85
Knight CG, Stephens T (1989) Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochem J 258:683–687
Ahmad M, King TA, Do-Kyeong K, Cha BH, Jongmin L (2002) Performance and photostability of xanthene and pyrromethene laser dyes in sol–gel phases. J Phys D Appl Phys 35:1473–1476
Bigdeli MA, Heravi MM, Mahdavinia GH (2007) Silica supported perchloric acid (HClO4-SiO2): a mild, reusable and highly efficient heterogeneous catalyst for the synthesis of 14-aryl or alkyl-14-Hdibenzo[a, j]xanthenes. J Mol Catal A Chem 275:25–29
Shaterian HR, Ghashang M, Hassankhani A (2008) One-pot synthesis of aryl 14H-dibenzo[a, j]xanthene leuco-dye derivatives. Dyes Pigments 76:564–568
Madhav JV, Reddy YT, Reddy PN, Reddy MN, Kuarma S, Crooks A, Rajitha B (2009) Cellulose sulfuric acid. An efficient biodegradable and recyclable solid acid catalyst for the one-pot synthesis of aryl 14H-dibenzo[a.j]xanthenes under solvent-free conditions. J Mol Catal A Chem 304:85–87
Shaterian HR, Ghashang M, Mir N (2007) Aluminium hydrogensulfate as an efficient and heterogeneous catalyst for preparation of aryl 14H-dibenzo[a, j]xanthene derivatives under thermal and solvent-free condition. ARKIVOC 15:1–10
Heravi MM, Bakhtiari K, Daroogheha Z, Bamoharram FF (2007) Facile heteropolyacid-promoted synthesis of 14-substituted-14-H-dibenzo[a, j]xanthene derivatives under solvent-free conditions. J Mol Catal A Chem 272:99–101
Khaligh NG (2012) Poly(4-vinylpyridinium) hydrogen sulfate: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions. Catal Sci Technol 2:2211–2215
Liu YH, Tao XY, Lei LQ, Zhang ZH (2009) Fluoroboric acid adsorbed on silica-gel catalyzed synthesis of 14-aryl-14Hdibenzo[a, j]xanthene derivatives. Synth Commun 39:580–589
Esmaeilpour M, Javidi J, Dehghani F, Dodeji FN (2014) Fe3O4@SiO2-imid-PMAn magnetic porous nanospheres as recyclable catalysts for the one-pot synthesis of 14-aryl- or alkyl-14Hdibenzo[a, j]xanthenes and 1,8-dioxooctahydroxanthene derivatives under various conditions. New J Chem 38:5453–5461
Prasad D, Nath M (2012) PEG-SO3H catalyzed, environmentally benign synthesis of 14-aryl 14Hdibenzo[a, j]xanthenes under solvent-free conditions. Catal Sci Technol 2:93–96
Naeimi H, Nazifi ZS (2014) Sulfonated diatomite as heterogeneous acidic nanoporous catalyst for synthesis of 14-aryl-14-H-dibenzo[a, j]xanthenes under green conditions. Appl Catal A Gen 477:132–140
Saghanezhad SJ, Nazari Y, Davod F (2016) Cucurbit[6]uril-OSO3H: a novel acidic nanocatalyst for the one-pot preparation of 14-aryl-14H-dibenzo[a, j]xanthenes and 1,8-dioxo-octahydro-xanthenes. RSC Adv 6:25525–25530
Ko S, Yao CF (2006) Heterogeneous catalyst: amberlyst-15 catalyzes the synthesis of 14-substituted-14H-dibenzo[a, j]xanthenes under solvent-free conditions. Tetrahedron Lett 47:8827–8829
Rezayati S, Erfani Z, Hajinasiri R (2015) Phospho sulfonic acid as efficient heterogeneous Brønsted acidic catalyst for one-pot synthesis of 14H-dibenzo[a, j]xanthenes and 1,8-dioxo-octahydro-xanthenes. Chem Pap 69:536–543
Allameh S, Davoodni A, Khojastehnezhad A (2012) An efficient and eco-friendly synthesis of 14-aryl-14H-dibenzo[a, j]xanthenes using H4[SiW12O40] as a heterogeneous and reusable catalyst under solvent-free conditions. Chin Chem Lett 23:17–20
Rostamizadeh S, Amani AM, Mahdavinia GH, Shadjou N (2009) Silica supported ammonium dihydrogen phosphate (NH4H2PO4/SiO2): a mild, reusable and highly efficient heterogeneous catalyst for the synthesis of 14-aryl-14-Hdibenzo[a, j]xanthenes. Chin Chem Lett 20:779–783
Rivera TS, Blanco MN, Pizzio LR, Romanelli GP (2012) Green catalytic synthesis of 14-aryl-14H-dibenzo[a, j]xanthenes using recyclable mesoporous zirconia modified with tungstophosphoric acid. Green Chem Lett Rev 5:433–437
Karimi AR, Zeinab DK, Marzie K (2014) Magnetite-sulfuric acid magnetic nanoparticles: preparation and application in synthesis of mono-, bis-, and tris-14Hdibenzo[a, j]xanthen-14-yl-arenes under solvent-free conditions. Synthesis 46:917–922
Fareghi-Alamdari R, Golestanzadeh M, Agend F, Zekri N (2013) Application of highly sulfonated single-walled carbon nanotubes. An efficient heterogeneous catalyst for the one-pot synthesis of 14-aryl-14H-dibenzo[a, j]xanthenes under solvent-free conditions. CR Chim 16:878–887
Naik MA, Sachdev D, Dubey A (2010) Sulfonic acid functionalized mesoporous SBA-15 for one-pot synthesis of substituted aryl-14H-dibenzoxanthenes and bis(indolyl)methanes. Catal Commun 11:1148–1153
Shaterian HR, Doostmohammadi R, Ghashang M (2008) Sodium hydrogen sulfate as effective and reusable heterogeneous catalyst for the one-pot preparation of 14H-[(Un)substituted phenyl]-dibenzo[a, j]xanthene leuco-dye derivatives. Chin J Chem 26:338–342
Baghbanian SM, Khanzad G, Vahdat SM, Tashakkorian H (2015) p-Sulfonic acid calix[4]arene as an efficient and reusable catalyst for the synthesis of acridinediones and xanthenes. Res Chem Intermed 41:9951–9966
Rajitha B, Sunil Kumar B, Thirupathi Reddy Y, Narsimha Reddy P, Sreenivasulu N (2005) Sulfamic acid: a novel and efficient catalyst for the synthesis of aryl-14H-dibenzo[a.j]xanthenes under conventional heating and microwave irradiation. Tetrahedron Lett 46:8691–8693
Shakibaei GI, Mirzaei P, Bazgir A (2007) Dowex-50 W promoted synthesis of 14-aryl-14H-dibenzo[a, j]xanthene and 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions. Appl Catal A Gen 325:188–192
Kundu K, Nayak S (2014) Camphor-10-sulfonic acid catalyzed condensation of 2-naphthol with aromatic/aliphatic aldehydes to 14-aryl/alkyl-14H-dibenzo[a, j]xanthenes. J Ser Chem Soc 79:1051–1058
Patil SB, Bhat RP, Samant SD (2006) Cation-exchange resins: efficient heterogeneous catalysts for facile synthesis of dibenzoxanthene from β-naphthol and aldehydes. Synth Commun 36:2163–2168
Nagarapu L, Baseeruddin M, Kumari NV, Kantevari S, Rudradas AP (2007) Efficient synthesis of aryl-14H-dibenzo[a.j]xanthenes using NaHSO4–SiO2 or 5%WO3/ZrO2 as heterogeneous catalysts under conventional heating in a solvent-free media. Synth Commun 37:2519–2525
Nandi M, Mondal J, Sarkar K, Yamauchi Y, Bhaumik A (2011) Highly ordered acid functionalized SBA-15: a novel organocatalyst for the preparation of xanthenes. Chem Commun 47:6677–6679
Rahmatpour A (2011) An efficient, high yielding, and eco-friendly method for the synthesis of 14-aryl- or 14-alkyl-14H-dibenzo[a, j]xanthenes using polyvinylsulfonic acid as a recyclable Brønsted acid catalyst. Monatsh Chem 142:1259–1263
Mahdavinia GH, Rostamizadeh S, Amani AM, Emdadi Z (2008) Ultrasound-promoted greener synthesis of aryl-14-H-dibenzo[a, j]xanthenes catalyzed by NH4H2PO4/SiO2 in water. Ultrason Sonochem 16:7–10
Mirjalili BBF, Bamoniri A, Akbari A (2011) Synthesis of 14-aryl or alkyl-14Hdibenzo[a, j]xanthenes promoted by Mg(HSO4)2. Chin Chem Lett 22:45–48
Karimi-Jaberi Z, Keshavarzi M (2010) Efficient one-pot synthesis of 14-substituted-14H-dibenzo[a, j]xanthenes using boric acid under solvent-free conditions. Chin Chem Lett 21:547–549
Khosropour AR, Khodaei MM, Moghannian H (2005) A facile, simple and convenient method for the synthesis of 14-alkyl or aryl-14-H-dibenzo[a, j]xanthenes catalyzed by pTSA in solution and solvent-free conditions. Synlett 6:955–958
Bigdeli MA, Heravi MM, Mahdavinia GH (2007) Wet cyanuric chloride catalyzed simple and efficient synthesis of 14-aryl- or -alkyl-14H-dibenzo[a, j]xanthenes. Catal Commun 8:1595–1598
Saini A, Kumar S, Sandhu JS (2006) A new LiBr-catalyzed, facile and efficient method for the synthesis of 14-alkyl or aryl-14H-dibenzo[a, j]xanthenes and tetrahydrobenzo[b]pyrans under solvent-free conventional and microwave heating. Synlett 12:1928–1932
Wang B, Li P, Zhang Y, Wang L (2010) FeCl3-catalyzed condensation of 2-naphthol and aldehydes under solvent-free reaction conditions. An efficient and green alternative for the synthesis of 14-aryl(alkyl)-14-H dibenzo[a, j]xanthenes. Chin J Chem 28:2463–2468
Su W, Yang D, Jin C (2008) Yb(OTf)3 catalyzed condensation reaction of b-naphthol and aldehyde in ionic liquids: a green synthesis of aryl-14H-dibenzo[a, j]xanthenes. Tetrahedron Lett 49:3391–3394
Kumar A, Sharma S, Maury RA, Sarkar J (2010) Diversity oriented synthesis of benzoxanthene and benzochromene libraries via one-pot, three-component reactions and their anti-proliferative activity. ACS Comb Sci 12:20–24
Cao Y, Yao C, Qin B, Zhang H (2013) Solvent-free synthesis of 14-aryl-14H-dibenzo[a, j]xanthenes catalyzed by recyclable and reusable iron(III) triflate. Res Chem Intermed 39:3055–3062
Soleimani E, Khodaei MM, Koshvandi ATK (2011) The efficient synthesis of 14-alkyl or aryl 14H-dibenzo[a, j]xanthenes catalyzed by bismuth(III) chloride under solvent-free conditions. Chin Chem Lett 22:927–930
Zolfigol MA, Moosavi-Zare AR, Arghavani-Hadi P, Zare A, Khakyzadeh V, Darvishi G (2012) WCl6 as an efficient, heterogeneous and reusable catalyst for the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes with high TOF. RSC Adv 2:3618–3620
Bhattacharya AK, Rana KC, Mujahid M, Sehar IS, Saxena AK (2009) Synthesis and in vitro study of 14-aryl-14Hdibenzo[a.j]xanthenes as cytotoxic agents. Bioorg Med Chem Lett 19:5590–5593
Mosaddegh E, Islami MR (2008) Synthesis of aryl 14H-dibenzo[a, j]xanthenes using zirconium(IV) oxide chloride as a catalyst. Org Prep Proc Int 40:586–589
Selvam NP, Shanthi G, Perumal PT (2007) Ceric-sulfate-catalyzed synthesis of 14-aryl- or 14-alkyl-14H-dibenzo[aj]xanthene under conventional heating and microwave irradiation. Can J Chem 85:989–995
Kantevari S, Chary MV, Das APR, Vuppalapati SVN, Lingaiah N (2008) Catalysis by an ionic liquid: highly efficient solvent-free synthesis of aryl-14-H-dibenzo[a.j]xanthenes by molten tetrabutylammonium bromide under conventional and microwave heating. Catal Commun 9:1575–1578
Zarei A, Hajipour AR, Khazdooz L (2010) The one-pot synthesis of 14-aryl or alkyl-14Hdibenzo[a,j]xanthenes catalyzed by P2O5/Al2O3 under microwave irradiation. Dyes Pigments 85:133–138
Madhav JV, Kumar VN, Someshwar P, Rajitha B (2008) A simple and convenient method for the synthesis of Aryl-14 H -dibenzo[a, j]xanthenes by using dipyridine copper chloride as Lewis acid catalyst. J Heterocycl Chem 45:119–121
Madhav JV, Kuarm BS, Rajitha B (2008) Dipyridine cobalt chloride: a novel and efficient catalyst for the synthesis of 14-aryl 14H-dibenzo[a, j]xanthenes under solvent-free conditions. ARKIVOC 2:204–209
Ding F-Q, An LT, Zou JP (2007) Iodine catalyzed microwave-assisted synthesis of 14-Aryl(Alkyl)-14H-dibenzo[a,j]xanthenes. Chin J Chem 25:645–648
Das B, Ravikanth B, Ramu R, Laxminarayana K, Rao BV (2006) Iodine catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo[a, j]xanthenes. J Mol Catal A Chem 255:74–77
Mirjalili BBF, Bamoniri A, Akbari A (2008) BF3·SiO2: an efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo[a, j]xanthenes. Tetrahedron Lett 49:6454–6456
Kumar PS, Kumar BS, Rajitha B, Reddy PN, Sreenivasulu N, Reddy YT (2006) A novel one pot synthesis of 14-aryl-14H-dibenzo[a, j]xanthenes catalyzed by SelectfluorTM under solvent free conditions. ARKIVOC 12:46–50
Bansal P, Chaudhary GR, Kaur N, Mehta SK (2015) An efficient and green synthesis of xanthene derivatives using CuS quantum dots as a heterogeneous and reusable catalyst under solvent free conditions. RSC Adv 5:8205–8209
Chaudhary GR, Bansal P, Kaur N, Mehta SK (2014) Recyclable CuO nanoparticles as heterogeneous catalysts for the synthesis of xanthenes under solvent free conditions. RSC Adv 4:49462–49470
Safaei-Ghomi J, Ghasemzadeh MA (2012) Zinc oxide nanoparticles, a highly efficient and readily recyclable catalyst for the synthesis of xanthenes. Chin Chem Lett 23:1225–1229
Haeri HH, Rezayati S, Nezhad ER, Darvishi H (2016) Fe2+ supported on hydroxyapatite-core-shell-γ-Fe2O3 nanoparticles: efficient and recyclable green catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives. Res Chem Intermed 42:4773–4784
Albadi A, Iravani N, Khoshakhlagh M (2012) A new, green and recyclable poly(4-vinylpyridine)-supported copper iodide nanoparticles catalyst for the synthesis of aryl-14H-dibenzo[a, j]xanthenes. Iran J Catal 2:85–89
Tabatabaeian K, Zanjanchi MA, Mamaghani M, Dadashi A (2015) Ruthenium anchored on multi-walled carbon nanotubes: an efficient and reusable catalyst for the synthesis of xanthenes. Res Chem Intermed 42:5049–5067
Shirini F, Abedini M, Seddighi M, Jolodar OG, Safarpoor M, Langroodi N, Zamani S (2014) Introduction of a new bi-SO3H ionic liquid based on 2,2-bipyridine as a novel catalyst forthe synthesis of various xanthene derivatives. RSC Adv 4:63526–63532
Shirini F, Yahyazadeh A, Mohammadi K (2014) One-pot synthesis of various xanthene derivatives using ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable catalyst under solvent free conditions. Chin Chem Lett 25:341–347
Gong K, Fang D, Wang HL, Zhou XL, Liu ZL (2008) The one-pot synthesis of 14-alkyl- or aryl-14H-dibenzo[a, j]xanthenes catalyzed by task-specific ionic liquid. Dyes Pigments 80:30–33
Das PJ, Das J (2015) Secondary amine based ionic liquid: an efficient catalyst for solvent free one pot synthesis of xanthenes and benzoxanthenes. RSC Adv 5:11745–11752
Dutta AK, Gogoi P, Borah R (2012) Synthesis of dibenzoxanthene and acridine derivatives catalyzed by 1,3-disulphonic acid imidazolium carboxylate ionic liquids. RSC Adv 4:41287–41291
Maleki B, Akbarzadeh E, Babaee S (2015) New basic ionic liquid from ethan-1,2-diylbis(hydrogen sulfate) and DBU (1,8- diazobicyclo[5.4.0]undec-7-ene) as an efficient catalyst for one-pot synthesis of xanthene derivatives. Dyes Pigments 123:222–234
Rahmati A (2010) A rapid and efficient method for the synthesis of 14H-dibenzo[a,j]xanthenes, aryl-5Hdibenzo[b.i]xanthene-5,7,12,14-(13H)-tetraones, and 1,8-dioxo-octahydroxanthenes by acidic ionic liquid. Chin Chem Lett 21:761–764
Rad-Moghadam K, Azimi SC (2012) Mg(BF4)2 doped in [BMIm][BF4]. A homogeneous ionic liquid-catalyst for efficient synthesis of 1,8-dioxo-octahydroxanthenes, decahydroacridines and 14-aryl-14-H-dibenzo[a, j]xanthenes. J Mol Catal A Chem 363–364:465–469
Wu H, Chen XM, Wan Y, Xin HQ, Xu HH, Yue CH, Ma R (2009) Synthesis and luminescence of 14-Aryl- or Alkyl-14H-dibenzo[a, j]xanthenes Catalyzed by 2–1′-Methylimidazolium-3-yl-1-ethyl Sulfate. Synth Commun 39:3762–3771
Khurana JM, Lumb A, Pandey A, Magoo D (2012) Green approaches for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones in aqueous media and under microwave irradiation in solventless conditions. Synth Commun 42:1796–1803
Liu YH, Li L (2012) Methanesulfonic acid-catalyzed one-pot synthesis of 12-aryl- or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives. J Heterocycl Chem 49:861–864
Wang HJ, Ren XQ, Zhang YY, Zhang ZH (2009) Synthesis of 12-aryl or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives catalyzed by dodecatungstophosphoric acid. J Braz Chem Soc 20:1939–1943
Li JT, Li YW, Song YL (2012) Efficient synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives catalyzed by p-dodecylbenzenesulfonic acid in aqueous media under ultrasound irradiation. Synth Commun 42:2161–2170
Khurana JM, Magoo D (2009) pTSA-catalyzed one-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones in ionic liquid and neat conditions. Tetrahedron Lett 50:4777–4780
Rama V, Kanagaraj K, Pitchumani K (2012) A multicomponent, solvent-free, one-pot synthesis of benzoxanthenones catalyzed by HY zeolite: their anti-microbial and cell imaging studies. Tetrahedron Lett 53:1018–1024
Bahrami K, Khodaei MM, Roostaei M (2014) The preparation and characterization of boehmite nanoparticles-TAPC: a tailored and reusable nanocatalyst for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones. New J Chem 38:5515–5520
Sadeghi B, Hassanabadi A, Taghvatalab E (2011) Nanoparticle silica supported sulfuric acid(NPs SiO2-H2SO4): a solid phase acidic catalyst for the one-pot synthesis of benzo[a]xanthen-11-one derivatives. J Chem Res 35:707–708
Maleki B, Barzegar S, Sepehr Z, Kermanian M, Tayebee R (2012) A novel polymeric catalyst for the one-pot synthesis of xanthene derivatives under solvent-free conditions. J Iran Chem Soc 9:757–765
Moghanian H, Mobinikhaledi A, Deinavizadeh M (2015) Efficient, one-pot synthesis of xanthene derivatives using boron sulphonic acid as a solid heterogeneous catalyst under solvent free conditions. Res Chem Intermed 41:4387–4394
Karimi N, Oskooie HA, Heravi MM, Tahershamsi L (2011) Caro’s acid-silica gel-catalyzed one-pot synthesis of 12-aryl-8,9,10,12- tetrahydrobenzo[a]xanthen-11-ones. Synth Commun 41:307–312
Heravi MM, Hashemi E, Beheshtiha YS, Kamjou K, Toolabi M, Hosseintash N (2014) Solvent-free multicomponent reactions using the novel N-sulfonic acid modified poly(styrene-maleic anhydride) as a solid acid catalyst. J Mol Catal A Chem 392:173–180
Vahdat S, Khaksar S (2015) Polyvinylpolypyrrolidone supported triflic acid (PVPP.OTf); an efficient and recyclable heterogeneous catalyst for one-pot condensation of b-naphthol, aldehydes, and cyclic 1,3-dicarbonyl compounds. Res Chem Intermed 41:4177–4186
Shaterian HR, Mohammadnia M (2013) Nanocrystalline TiO2-HClO4 catalyzed three-component preparation of derivatives of 1-amidoalkyl-2-naphthol, 1-carbamato-alkyl-2-naphthol, 1-(a-aminoalkyl)-2-naphthol, and 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one. Res Chem Intermed 39:4221–4237
Zhang ZH, Wang HJ, Ren XQ, Zhang YY (2009) A facile and efficient method for synthesis of xanthone derivatives catalyzed by HBF4/SiO2 under solvent-free conditions. Monatsh Chem 140:1481–1483
Chen W, Peng XW, Zhong LX, Li Y, Sun RC (2015) Lignosulfonic acid: a renewable and effective biomass-based catalyst for multicomponent reactions. ACS Sustain Chem Eng 3:1366–1373
Pawar PB, Jadhav SD, Deshmukh MB, Patil S (2014) Citric acid as a mild and inexpensive organocatalyst for synthesis of tetrahydrobenzo[a]xanthen-11-ones and dibenzo[a, j]xanthenes under solvent-free condition. Ind J Chem 14:1185–1193
Shaterian HR, Rigi F (2014) New applications of cellulose-SO3H as a bio-supported and biodegradable catalyst for the one-pot synthesis of some three-component reactions. Res Chem Intermed 40:2983–2999
Wan Y, Zhang XX, Wang C, Zhao LL, Chen LF, Liu GX (2013) The first example of glucose-containing Brønsted acid synthesis and catalysis: efficient synthesis of tetrahydrobenzo[a]xanthens and -tetrahydrobenzo[a]acridines in water. Tetrahedron 69:3947–3950
Ma J, Zhong L, Peng X, Sun R (2016) D-Xylonic acid: a solvent and an effective biocatalyst for a three-component reaction. Green Chem 18:1738–1750
Wang RZ, Zhang LF, Cui ZS (2009) Iodine-catalyzed synthesis of 12-aryl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one derivatives via multicomponent reaction. Synth Commun 39:2101–2107
Sun XJ, Zhou JF, Zhao PS (2011) Molecular iodine-catalyzed one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under microwave irradiation. J Heterocycl Chem 48:1347–1350
Foroughifar N, Mobinikhaledi A, Moghanian H, Mozafari R, Esfahani HRM (2011) Ammonium chloride-catalyzed one-pot synthesis of tetrahydrobenzo[a]xanthen-11-one derivatives under solvent-free conditions. Synth Commun 41:2663–2673
Kidwai M, Mishra A, Jahan NK (2012) A novel method for the synthesis of tetrahydrobenzo[a]xanthen-11-one derivatives using cerium(III) chloride as a highly efficient catalyst. C R Chim 15:324–330
Nandi GC, Samai S, Kumar R, Singh MS (2009) An efficient one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under solvent free condition. Tetrahedron 65:7129–7134
Li J, Tang W, Lu L, Su W (2008) Strontium triflate catalyzed one-pot condensation of b-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds. Tetrahedron Lett 49:7117–7120
Sharma RK, Khajuria R, Kapoor KK (2014) Alum-catalyzed domino synthesis of 12-substituted-8,9,10,12-tetrahydrobenzoxanthen-11-ones under Solvent-free conditions. Synth Commun 44:3538–3551
Tabatabaeian K, Khorshidi A, Mamaghani M, Dadashi A, Jalali MK (2011) One-pot synthesis of tetrahydrobenzo[a]xanthen-11-one derivatives catalyzed by ruthenium chloride hydrate as a homogeneous catalyst. Can J Chem 89:623–627
Mirjalili FBB, Bamoniri A, Zamani L (2012) Nano-TiCl4/SiO2: an efficient and reusable catalyst for the synthesis of tetrahydrobenzo[a]xanthen-11-ones. Lett Org Chem 9:338–343
Farhad Shirini F, Akbari-Dadamahaleh S, Mohammad-Khah A, Aliakbar AR (2013) Rice husk: a mild, efficient, green and recyclable catalyst for the synthesis of 12-Aryl-8, 9, 10, 12-tetrahydro[a]xanthene-11-ones and quinoxaline derivatives. C R Chim 16:207–216
Taghavi-Khorasani F, Davoodnia A (2015) A fast and green method for synthesis of tetrahydrobenzo[a]xanthene-11-ones using Ce(SO4)2·4H2O as a novel, reusable, heterogeneous catalyst. Res Chem Intermed 41:2415–2425
Oskooie HA, Heravi MM, Karimi N, Kohansal G (2011) Cu/SiO2-catalyzed one-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones under solvent-free conditions. Synth Commun 41:2763–2768
Kaur B, Parmar A, Kumar H (2012) Manganese Perchlorate-catalyzed greener synthesis of 12-aryl or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives under ultrasonication. Synth Commun 42:447–453
Gao S, Tsai CH, Yao CF (2009) A simple and green approach for the synthesis of tetrahydrobenzo[a]-xanthen-11-one derivatives using tetrabutylammonium fluoride in water. Synlett 6:949–954
Akondi AM, Kantam ML, Trivedi R, Sreedhar B, Buddana SK, Prakasham RS, Bhargava S (2014) Formation of benzoxanthenones and benzochromenones via cerium-impregnated-MCM-41 catalyzed, solvent-free, three component reaction and their biological evaluation as anti-microbial agents. J Mol Catal A Chem 386:49–60
Mohammadi R, Eidi E, Ghavami M, Kassaee MZ (2014) Chitosan synergistically enhanced by successive Fe3O4 and silver nanoparticles as a novel green catalyst in one-pot, three component synthesis of tetrahydrobenzo[a]xanthene-11-ones. J Mol Catal A Chem 393:309–316
Khazaei A, Zolfigol MA, Moosavi-Zare AR, Zare A, Khojasteh M, Asgari Z, Khakyzadeh V, Khalafi-Nezhad A (2012) Organocatalyst trityl chloride efficiently promoted the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones by in situ formation of carbocationic system in neutral media. Catal Commun 20:54–57
Iniyavan P, Sarveswari S, Vijayakumar V (2015) Microwave-assisted clean synthesis of xanthenes and chromenes in [bmim][PF6] and their antioxidant studies. Res Chem Intermed 41:7413–7426
Fang D, Yang JM, Cao YF (2013) Synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones catalyzed by biodegradable ionic liquid. Res Chem Intermed 39:1745–1751
Kundu D, Majee A, Hajra A (2011) Task-specific ionic liquid-catalyzed efficient microwave-assisted synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo [a]xanthen-11-ones ones under solvent-free conditions. Green Chem Lett Rev 4:205–209
Zolfigol MA, Khakyzadeh V, Moosavi-Zare AR, Zare A, Azimi SB, Asgari Z, Hasaninejad A (2012) Preparation of various xanthene derivatives over sulfonic acid-functionalized imidazolium salts (SAFIS) as novel, highly efficient and reusable catalysts. C R Chim 15:719–736
Singh H, Kumari S, Khurana JM (2014) A new green approach for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives using task specific acidic ionic liquid [NMP]H2PO4. Chin Chem Lett 25:1336–1340
Heydari R, Shahrekipour F (2015) One-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones by using of neutral and efficient organocatalysts under solvent-free conditions. Res Chem Intermed 41:4581–4586
Yadav S, Khurana JM (2013) An efficient synthesis of novel 3-hydroxy-12-arylbenzo[a]xanthen-11-ones and 5,12-diarylxantheno[2,1-a]xanthene-4,12-diones using pTSA in [bmim]BF4. Can J Chem 91:698–703
Khurana JM, Nand B, Sneha (2011) An efficient and convenient approach for the synthesis of novel 2-hydroxy-12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones using p-toluenesulfonic acid in ethanol and ionic liquid. J Heterocycl Chem 48:1388–1392
Khurana JM, Chaudhary A, Lumb A, Nand B (2012) Efficient one-pot syntheses of dibenzo[a, i]xanthene-diones and evaluation of their antioxidant activity. Can J Chem 90:739–746
Wu L, Zhang J, Fang L, Yang C, Yan F (2010) Silica chloride catalyzed synthesis of 14-aryl-14H-dibenzo[a, i]xanthene-8,13-diones. Dyes Pigments 86:93–96
Rahmatpour A (2013) Polystyrene-supported GaCl3 as a highly efficient and reusable heterogeneous Lewis acid catalyst for the three-component synthesis of benzoxanthene derivatives. Monatsh Chem 144:1205–1212
Srinivas V, Rao VR (2012) Facile, one-pot, three-component synthesis of benzo[a]naphthacene-8,13-diones. Synth Commun 42:388–393
Liu D, Xu D, Gao J, Zhou S (2014) p-Toluenesulfonic acid catalyzed synthesis of 14-aryl-14H-dibenzo[a, i]xanthene-8,13-dioness. Chem Sci Trans 3:455–459
Ghasemzadeh MA, Azimi-Nasrabad M (2016) Nano-Fe3O4-encapsulated silica particles bearing sulfonic acid groups as a magnetically separable catalyst for the green and efficient synthesis of 14-aryl-14H-dibenzo[a, i]xanthene-8,13-dione derivatives. Res Chem Intermed 42:1057–1069
Safaei-Ghomi J, Eshteghal F (2017) Nano-Fe3O4/PEG/succinic anhydride: a novel and efficient catalyst for the synthesis of benzoxanthenes under ultrasonic irradiation. Ultrason Sonochem 38:488–495
Yang LM, Yin ZK, Wu LQ (2012) H4SiW12O40: an efficient catalyst for the synthesis of new spiro[dibenzo[a, i]xanthene-14,30-indoline]-20,8,13-triones. Chin Chem Lett 23:265–268
Yang X, Yang L, Wu L (2012) [Hmim][HSO4]: an efficient and reusable catalyst for the synthesis of spiro[dibenzo[a, i] xanthene-14,3′-indoline]-2′,8,13-triones and spironaphthopyran[2,3-d]pyrimidine-5,3′-indolines. Bull Korean Chem Soc 33:714–716
Kefayati H, Bazargard SJ, Vejdansefat P, Shariati S, Kohankar AM (2016) Fe3O4@MCM-41-SO3H@[HMIm][HSO4]: an effective magnetically separable nanocatalyst for the synthesis of novel spiro[benzoxanthene-indoline]diones. Dyes Pigm 125:309–315
Mohr SJ, Chirigos MA, Fuhrman FS, Pryor JW (1975) Pyran copolymer as an effective adjuvant to chemotherapy against a murine leukemia and solid tumor. Cancer Res 35:3750–3754
Dell C, Smith C Antiproliferative derivatives of 4H-naphtho [1, 2-b] Pyran and process for their preparation. EP537949. 1993:21
Smith PW, Sollis SL, Howes PD, Cherry PC, Starkey ID, Cobley KN, Weston H, Scicinski J, Merritt A, Whittington A (1998) Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. J Med Chem 41:787–789
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) A facile one-pot green synthesis and antibacterial activity of 2-amino-4H-Pyrans and 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes. Eur J Med Chem 44:3805–3809
Chattapadhyay TK, Dureja PJ (2006) Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J Agric Food Chem 54:2129–2133
Mineeva IV (2015) Cyclopropanol methodology in the synthesis of (4R)- and (4S)-4-methyltetrahydro-2H-pyran-2-ones. Application in the synthesis of insect pheromones with methyl-branched carbon skeleton. Russ J Org Chem 51:341–351
Bianchi G, Tava A (1987) Synthesis of (2R)-(+)-2,3-Dihydro-2,6-dimethyl-4H-Pyran-4-one, a homologue of pheromones of a species in the Hepialidae family. Agric Biol Chem 51:2001–2002
Armesto D, Horspool WM, Martin N, Ramos A, Seoane C (1989) Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3,5-dicyano-6-phenyl-4H-Pyrans. J Org Chem 54:3069–3072
Awuah SG, Polreis J, Prakash J, Qiao Q, You Y (2011) New pyran dyes for dye-sensitized solar cells. J Photochem Photobio A Chem 224:116–122
Ahmed MM, El-Saghier MB, Naili BK, Rammash NA, Saleh Khaled MK (2007) Synthesis and antibacterial activity of some new fused chromenes. ARKIVOC 16:83–91
Tilak R, Richa KB, Rakesh KS, Vivek G, Deepak S, Mohan PSI (2009) Mechanism of unusual formation of 3-(5-phenyl-3H-[1,2,4]dithiazol-3yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides and their antimicrobial evaluation. Eur J Med Chem 44:3209–3216
Okram MS, Nepram SD, Dhanaraj ST, Gurumayum JS (2010) Novel 3alkanoyl/aroyl/heteroaroyl-2H-chromene-2-thiones: synthesis and evaluation of their antioxidant activities. Eur J Med Chem 45:2250–2257
Kumar A, Maurya RA, Sharma S, Ahmad P, Singh AB, Bhatia G, Srivastava AK (2009) Pyranocoumarins: a new class of anti-hyperglycemic and anti-dyslipidemic agents. Bioorg Med Chem Lett 19:6447–6451
Mehrabi H, Kamali N (2012) Efficient and eco-friendly synthesis of 2-amino-4H-chromene derivatives using catalytic amount of tetrabutylammonium chloride (TBAC) in water and solvent-free conditions. J Iran Chem Soc 9:599–605
Eshghi H, Damavandi S, Zohuri GH (2011) Efficient one-pot synthesis of 2-amino-4H-chromenes catalyzed by ferric hydrogen sulfate and Zr-Based Catalysts of FI. Synth React Inorg Met Org Nano-Met Chem 41:1067–1073
Meng XY, Wang HJ, Wang CP, Zhang ZH (2011) Disodium hydrogen phosphate as an efficient and cheap catalyst for the synthesis of 2-aminochromenes. Synth Commun 41:3477–3484
Shekhar AC, Kumar AR, Sathaiah G, Raju K, Rao PS, Sridhar M, Sridhar B (2012) An efficient one-pot synthesis of substituted 1h-naphtho[2,1-b]pyrans and 4H-1-Benzopyrans (Chromenes) under solvent-free microwave-irradiation conditions. Helv Chim Acta 95:502–508
Sadjadi S, Heravi MM, Zadsirjan V, Ebrahimizadeh M (2017) SBA-15@methenamine-HPA: a novel, simple, and efficient catalyst for one-pot three-component synthesis of 2-amino-4H-chromene derivatives in aqueous medium. Res Chem Intermed 43:5467–5483
Sadeghi B, Zarepour I (2015) Nano-sawdust–BF3 as a new, cheap, and effective nano catalyst for one-pot synthesis of 2-amino benzo[h]chromene derivatives. J Nanostruct Chem 5:305–311
Sunil Kumar B, Srinivasulu N, Udupi RH, Rajitha B, Thirupathi Reddy Y, Narsimha Reddy P, Kumar PS (2006) Efficient synthesis of benzo[g]-and benzo[h]chromene derivatives by one-pot three-component condensation of aromatic aldehydes with active methylene compounds and naphthols. Russ J Org Chem 42:1813–1815
Balalaie S, Ramezanpour S, Bararjanian M, Gross JH (2008) DABCO-catalyzed efficient synthesis of naphthopyran derivatives via one-pot three-component condensation reaction at room temperature. Synth Commun 38:1078–1089
Heravi MM, Hosseinnejad T, Faghihi Z, Shiri M, Vazinfard M (2017) Synthesis of 2-amino-3-cyano 4-H-chromenes containing quinoline in water: computational study on substituent effects. J Iran Chem Soc 14:823–832
Mobinikhaledi A, Moghanian H, Sasani F (2011) Synthesis and reactivity in inorganic, microwave-assisted one-pot synthesis of 2-amino-2-chromenes using piperazine as a catalyst under solvent-free conditions. Synth React Inorg Met Org Nano-Met Chem 41:262–265
Dekamin MG, Eslami M (2014) Highly efficient organocatalytic synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans under mechanochemical ball milling. Green Chem 16:4914–4921
Dekamin MG, Alikhani M, Javanshir S (2016) Organocatalytic clean synthesis of densely functionalized 4H-pyrans by bifunctional tetraethylammonium 2-(carbamoyl)benzoate using ball milling technique under mild conditions. Green Chem Lett Rev 9:96105
Kundu SK, Bhaumik A (2015) A triazine-based porous organic polymer: a novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes. RSC Adv 5:32730–32739
Saha A, Payra S, Banerjee S (2015) On water synthesis of pyran–chromenes via a multicomponent reactions catalyzed by fluorescent t-ZrO2 nanoparticles. RSC Adv 5:101664–101671
Albadi J, Alihoseinzadeh A, Mansournezhad A, Kaveiani L (2015) Novel metal oxide of CuO–ZnO nanocatalyst efficiently catalyzed the synthesis of 2-amino-4H-chromenes in water. Synth Commun 45:485–493
Tajbakhsh M, Kariminasab M, Alinezhad H, Hosseinzadeh R, Rezaee P, Tajbakhsh M, Gazvini HJ, Azizi Amiri M (2012) Nano silicabonded aminoethylpiperazine: a highly efficient and reusable heterogeneous catalyst for the synthesis of 4Hchromene and 12Hchromeno[2,3d]pyrimidine derivatives. J Iran Chem Soc 12:1405–1414
Karmakar B, Nandi R (2016) A green route towards substituted 2-amino-4H-chromenes catalyzed by an organobase (TBD) functionalized mesoporous silica nanoparticle without heating. Res Chem Intermed. https://doi.org/10.1007/s11164-016-2755-9
Maleki B, Sheikh S (2015) Nano polypropylenimine dendrimer (DAB-PPI-G1): as a novel nano basic-polymer catalyst for one-pot synthesis of 2-amino-2-chromene derivatives. RSC Adv 5:42997–43005
Jiang L, Yu H (2014) Enzymatic promiscuity: “Amano” lipase AS-catalysed synthesis of naphthopyran derivatives in anhydrous media. Chem Res Chin 30:396–399
Shinde S, Damate S, Morbale S, Patil M, Patil SS (2017) Aegle marmelos in heterocyclization: greener, highly efficient, one-pot three-component protocol for the synthesis of highly functionalized 4H-benzochromenes and 4H-chromenes. RSC Adv 7:7315–7328
Qareaghaj OH, Mashkouri S, Naimi-Jamal MR, Kaupp G (2014) Ball milling for the quantitative and specific solvent-free Knoevenagel condensation + Michael addition cascade in the synthesis of various 2-amino-4-aryl-3-cyano-4H-chromenes without heating. RSC Adv 4:48191–48201
Naimi-Jamal MR, Mashkouri S, Sharifi A (2010) An efficient, multicomponent approach for solvent-free synthesis of 2-amino-4H-chromene scaffold. Mol Div 14:473–477
Sheibani H, Saidi K, Abbasnejad M, Derakhshani A, Mohammadzadeh I (2016) A convenient one-pot synthesis and anxietic activity of 3-cyano-2(1H)iminopyridines and halogen derivatives of benzo[h]chromenes. Arabian J Chem 9:S901–S906
Wang X-S, Shi DQ, Yu HZ, Wang GF, Tu SG (2004) Synthesis of 2aminochromene derivatives catalyzed by KF/Al2O3. Synth Commun 34:509–514
Albadi J, Mansournezhad A, Darvishi-Paduk M (2013) Poly(4-vinylpyridine): as a green, efficient and commercial available basic catalyst for the synthesis of chromene derivatives. Chin Chem Lett 24:208–210
Shaterian HR, Mohammadnia M (2013) Mild preparation of 2-amino-3-cyano-4-aryl-4H-benzo[h]chromenes and 2-amino-3-cyano-1-aryl-1H-benzo[f]chromenes, under solvent-free conditions, catalyzed by recyclable basic ionic liquids. Res Chem Intermed 41:1301–1313
Shaikh MA, Farooqui M, Abed S (2019) Novel task-specific ionic liquid [Et2NH(CH2)2CO2H][AcO] as a robust catalyst for the efficient synthesis of some pyran-annulated scaffolds under solvent-free conditions. Res Chem Intermed 45:1595–1617
Warekar PP, Patil PT, Patil KT, Jamale DK, Kolekar GB, Anbhule PV (2017) PTSA-catalyzed straightforward novel approach for the synthesis of 1,2-bis(4-nitrophenyl)-1H-benzo[f]chromen-3-amine and the evaluation of their antituberculosis activity. Res Chem Intermed 43:4115–4127
Olyaei A, Shahsavari MS, Sadeghpour M (2017) Organocatalytic approach toward the green one-pot synthesis of novel benzo[f]chromenes and 12H-benzo[5,6]chromeno[2,3-b]pyridines. Res Chem Intermed 44:943–956
Hosseinnia R, Mamaghani M, Tabatabaeian K, Shirini F, Rassa M (2012) An expeditious regioselective synthesis of novel bioactive indole-substituted chromene derivatives via one-pot three-component reaction. Bioorg Med Chem Lett 22:5956–5960
Wan Y, Wang C, Wang H, Zhao L, Zhang X, Shi J, Wu H (2014) Efficient One-Pot Syntheses of 7-Alkyl-6H,7H-naphtho[1,2:5,6]pyrano-[3,2-c]chromen-6-ones by 1-Methyl-3-(2-(sulfooxy)ethyl)-1H-imidazol-3-ium Chloride. J Heterocycl Chem 51:1293–1297
Ma W, Wang X, Yan F, Wu L, Wang Y (2010) Reusable melamine trisulfonic acid-catalyzed three-component synthesis of 7-alkyl-6H,7H-naphtho[1′,2′:5,6]pyrano[3,2-c]chromen-6-ones. Monatsh Chem 142:163–167
Nikpassand M, Leila ZF, Zahra G, Jafarian Z (2018) Potassium 2-oxoimidazolidine-1,3-diide as a novel catalyst for grind synthesis of pyrano[4,3-b]chromenone. J Chil Chem Soc 63:41965–44199
Bhattacharjee S, Gattu R, Khan AT (2018) Triethylamine-mediated one-pot synthesis of benzo[f]chromene derivatives. ChemistrySelect 3:4760–4763
Mashraqui SH, Patil MB, Mistry HD, Ghadigaonkar S, Meetsma A (2004) A three-component reaction of phenol, aldehyde, and active methylene substrate under lewis acid catalysis: successful trapping ofo-quinone methide to afford benzopyran systems. Chem Lett 33:1058–1059
Devi KM, Chanu LG, Chanu IS, Singh OM (2014) One-pot synthesis of 1H-naphtho[2,1-b]pyran derivatives under solvent- free conditions. Lett Org Chem 11:743–747
Kumar KP, Satyanarayana S, Reddy PL, Narasimhulu G, Ravirala N, Reddy BVS (2012) Iodine-catalyzed three-component one-pot synthesis of naphthopyranopyrimidines under solvent-free conditions. Tetrahedron Lett 53:1738–1741
Mohaqeq M, Safaei-Ghomi J, Shahbazi-Alavi H (2015) ZrOCl2/nano TiO2 as an efficient catalyst for the one pot synthesis of naphthopyranopyrimidines under solvent-free conditions. Acta Chim Slov 62:967–972
Khurana JM, Lumb A, Chaudhary A, Nand B (2013) Synthesis and in vitro evaluation of antioxidant activity of diverse naphthopyranopyrimidines, diazaanthra[2,3-d][1,3]dioxole-7,9-dione and tetrahydrobenzo[a]xanthen-11-ones. RSC Adv 3:1844–1854
Singh M, Nandi G, Samai S (2010) First InCl3-catalyzed, three-component coupling of aldehydes, β-naphthol, and 6-amino-1,3-dimethyluracil to functionalized naphthopyranopyrimidines. Synlett 07:1133–1137
Cimarellin C, Fratoni D, Palmier G (2011) Novel stereoselective synthesis of 2,3-dihydro-1H-benzo[f]chromen-3-amine derivatives through a one-pot three component reaction. Tetrahedron Asymmetry 22:1542–1547
Nizami TA, Hua R (2018) Synthesis of 3 H -naphtho[2.1- b]pyran-2-carboxamides from cyclocoupling of β-naphthol, propargyl alcohols and isocyanide in the presence of Lewis acids. Tetrahedron 74:3776–3780
Yadav JS, Subba Reddy BV, Biswas SK, Sengupta S (2009) Gallium(III) chloride-catalyzed three-component coupling of naphthol, alkyne and aldehyde: a novel synthesis of 1,3-disubstituted-3H-benzo[f] chromenes. Tetrahedron Lett 50:5798–5801
Asadi S, Mohammadi Ziarani G, Rahimifard M, Abolhassani Soorki A (2014) A green one-pot synthesis of spiron/aphthopyrano[1,2-b]indeno-7,3′-indolines. Res Chem Intermed 41:6219–6227
Kong D, Lu G, Wu M, Shi Z, Li Q (2017) One-pot, catalyst-free synthesis of spiro[dihydroquinoline-naphthofuranone] compounds from isatins in water triggered by hydrogen bonding effects. ACS Sustain Chem Eng 5:3465–3470
Mohammadi Ziarani G, Lashgari N, Faramarzi S, Badiei A (2014) Efficient synthesis of spironaphthopyrano [2,3-d]pyrimidine-5,3′-indolines under solvent-free conditions catalyzed by SBA-Pr-SO3H as a nanoporous acid catalyst. Acta Chim Slov 61:574–579
Heravi M, Zakeri M, Moharami A (2012) Versatile three-component procedure for combinatorial synthesis of spiro-oxindoles with fused chromenes catalysed by l-proline. J Chem Sci 124:865–869
Shanthi G, Subbulakshmi G, Perumal PT (2007) A new InCl3-catalyzed, facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions. Tetrahedron 63:2057–2063
Heravi MR, Norouzy F (2017) One-pot, four-component synthesis of novel biologically important indeno[2,3-b]quinoxaline]-3-carbonitriles in fluoro-alcohols under ultrasound irradiation. Res Chem Intermed 43:4265–4282
Khojasteh-Khosro S, Shahbazi-Alavi H (2019) Preparation of spirooxindoles catalyzed by nano-Co3S4 under microwave irradiations. J Chem Res 43:107–111
Mathew BP, Kumar A, Sharma S, Shukla PK, Nath M (2010) An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo- and naphtho-1,3-oxazine derivatives. Eur J Med Chem 45:1502–1507
Zanatta N, Squizani AMC, Fantinel L, Nachtigall FM, Borchhardt DM, Bonacorso HG, Martins MAP (2005) Synthesis of N-substituted 6-Trifluoromethyl-1,3-oxazinanes. J Braz Chem Soc 16:1255–1261
Zhang P, Terefenko EA, Fensome A, Wrobel J, Winneker R, Zhang Z (2003) Novel 6-Aryl-1,4-dihydrobenzo[d][1,3]oxazine-2-thiones as potent, selective, and orally active nonsteroidal progesterone receptor agonists. Bioorg Med Chem Lett 13:1313–1316
Kuehne ME, Konopka A (1962) Dihydro-1,3-oxazines as antitumor agents. J Med Chem 5:257–280
Azizian J, Yadollahzadeh K, Delbari AS, Ghanbari MM (2012) An efficient Biginelli one-pot synthesis of new naphthalene-condensed oxazine derivatives under microwave-assisted conditions. Monatsh Chem 143:1417–1420
Ghomi JS, Zahedi S, Ghasemzadeh MA (2012) An efficient green route for the preparation of naphthoxazinones, applying a three-component one-pot condensation reaction under solvent-free conditions. Iran J Catal 2(1):27–30
Kumar A, Saxena A, Dewan M, De A, Mozumdar S (2011) Recyclable nanoparticulate copper mediated synthesis of naphthoxazinones in PEG-400: a green approach. Tetrahedron Lett 52(38):4835–4839
Dharma Rao GB, Kaushik MP, Halve AK (2012) An efficient synthesis of naphtha[1,2-e]oxazinone and 14-substituted-14H-dibenzo[a, j]xanthene derivatives promoted by zinc oxide nanoparticle under thermal and solvent-free conditions. Tetrahedron Lett 53:2741–2744
Safaei Ghomi J, Zahedi S, Ghasemzadeh MA (2014) AgI nanoparticles as a remarkable catalyst in the synthesis of (amidoalkyl)naphthol and oxazine derivatives: an eco-friendly approach. Monatsh Chem 145:1191–1199
Salunkhe NG, Ladole CA, Thakare NV, Aswar AS (2017) MgFe2O4@SiO2–SO3H: an efficient, reusable catalyst for the microwave-assisted synthesis of benzoxazinone and benzthioxazinone via multicomponent reaction under solvent free condition. Res Chem Intermed 44:355–372
Basavegowda N, Somai Magar KB, Mishra K, Lee YR (2014) Green fabrication of ferromagnetic Fe3O4nanoparticles and their novel catalytic applications for the synthesis of biologically interesting benzoxazinone and benzthioxazinone derivatives. New J Chem 38:5415–5420
Bazgir A, Dabiri M, Delbari A (2007) A novel three-component, one-pot synthesis of 1,2-dihydro-1-aryl-naphtho[1,2-e][1,3]oxazine-3-one derivatives under microwave-assisted and thermal solvent-free conditions. Synlett 5:0821–0823
Sharma M, Manohar S, Rawat DS (2012) Lewis acid catalyzed synthesis of 1-aryl-1,2-dihydro-naphtho[1,2-e][1,3]oxazin-3-ones under solvent free conditions: a mechanistic approach. J Heterocycl Chem 49:589–595
Kantevari S, Vuppalapati SVN, Bantu R, Nagarapu L (2010) An efficient one-pot three component synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-ones using montmorillonite k10 under solvent free conditions. J Heterocycl Chem 47:313–317
Hajra A, Kundu D, Majee A (2009) An efficient one-pot synthesis of naphthoxazinones by a three-component coupling of naphthol, aldehydes, and urea catalyzed by zinc triflate. J Heterocycl Chem 46:1019–1022
Chaskar A, Vyavhare V, Padalkar V, Phatangare K, Deokar H (2011) An environmentally benign one-pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-one derivatives catalysed by phosphomolybdic acid. J Ser Chem Soc 76:21–26
Harichandran G, Parameswari P, Shanmugam P (2018) A one-pot multicomponent synthesis of naphthoxazin-3-one derivatives using Amberlite IRA-400 Cl resin as green catalyst. Lett Org Chem 15:600–605
Gupta A, Kour D, Gupta VK, Kapoor KK (2016) Graphene oxide mediated solvent-free three component reaction for the synthesis of 1-amidoalkyl-2-naphthols and 1,2-dihydro-1-arylnaphth[1,2- e][1,3]oxazin-3-ones. Tetrahedron Lett 57:4869–4872
Sabitha G, Arundhathi K, Sudhakar K, Sastry BS, Yadav JS (2010) A novel three-component one-pot reaction involving β-naphthol, aldehydes, and urea promoted by TMSCl/NaI. J Heterocycl Chem 47:272–275
Dong F, Yang Li-fang, Jin-ming Y (2012) Synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-one catalyzed by pyridinium-based ionic liquid. Res Chem Intermed 39:2505–2512
Zhu X, Lee YR (2012) RuCl2(PPh3)3-catalyzed facile one-pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-ones and 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-thiones. Bull Korean Chem Soc 33:3831–3834
Kumar A, Gupta MK, Kumar M (2012) Micelle promoted supramolecular carbohydrate scaffold-catalyzed multicomponent synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthols derivatives in aqueous medium. RSC Adv 2:7371
Lei M, Ma L, Hu L (2011) Highly chemoselective condensation of β-naphthol, aldehyde, and urea catalyzed by thiamine hydrochloride. Synth Commun 41:3424–3432
Shafiee M, Khosropour AR, Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Mirkhani V, Khavasi HR (2012) Synthesis of trans-1,3-diaryl-2-(5-methylisoxazol-3-yl)-2,3-dihydro-1H-naphtho[1,2-e][1,3]oxazines via bismuth(III)-catalyzed one-pot pseudo-four component reaction. Mol Div 16:727–735
Turgut Z, Pelit E, Köycü A (2007) Synthesis of new 1,3-disubstituted-2,3-dihydro-1H-naphth[1,2-e][1,3]oxazines. Molecules 12:345–352
Dutta AK, Gogoi P, Saikia MP, Borah R (2016) Development of environmentally benign methods towards the synthesis of anti-2,3-dihydro-1,2,3-trisubstituted-1H-naphth[1,2-e][1,3]oxazines using brønsted acidic catalysts. Catal Lett 146:902–908
Dutta AK, Gogoi P, Borah R (2017) Triphenylsulfophosphonium chlorometallates as efficient heterogeneous catalysts for the three-component synthesis of 2,3-dihydro-1,2,3-trisubstituted-1H-naphth[1,2-e][1,3]oxazines. Polyhedron 123:184–191
Sapkal SB, Shelke KF, Shingate BB, Shingare MS (2010) An efficient one-pot strategies for the synthesis of [1,3] oxazine derivatives. J Kor Chem Soc 54:437–442
Lohar T, Mane A, Kamat S, Salunkhe R (2018) A versatile water-stable fluorine containing organometallic lewis acid used in green synthesis of 1,3-oxazine scaffolds at room temperature. Polycyclc Aromat Comp. https://doi.org/10.1080/10406638.2018.1538057
Azad S, Mirjalili BBF (2018) One-pot solvent-free synthesis of 2,3-dihydro-2-substituted-1H-naphtho[1,2-e][1,3]oxazine derivatives using Fe3O4@nano-cellulose/TiCl as a bio-based and recyclable magnetic nano-catalyst. Mol Diver 23:413–420
Reddy MV, Lim KT, Kim JT, Jeong YT (2012) Ultrasound-assisted one-pot synthesis of 1,3-oxazine derivatives catalysed by BF3–SiO2 under neat conditions. J Chem Res 36:398–401
Dhakane VD, Gholap SS, Deshmukh UP, Chavan HV, Bandgar BP (2014) An efficient and green method for the synthesis of [1,3]oxazine derivatives catalyzed by thiamine hydrochloride (VB1) in water. C R Chim 17:431–436
Ganesan S, Rajendran N, Sundarakumar S, Ganesan A, Pemiah B (2013) β-Naphthol in glycerol: a versatile pair for efficient and convenient synthesis of aminonaphthols, naphtho-1,3-oxazines, and benzoxanthenes. Synthesis 45:1564–1568
Nongrum R, Kharkongor M, Nongthombam GS, Rani JWS, Rahman N, Kharmawlong GK, Nongkhlaw R (2019) [1,3]oxazines: green synthesis by sonication using a magnetically-separable basic nano-catalyst and investigation of its activity against the toxic effect of a pesticide on the morphology of blood cells. Environ Chem Lett 17:1325–1331
Sadaphal SA, Sonar SS, Shingate BB, Shingare MS (2010) Water mediated synthesis of various [1,3]oxazine compounds using alum as a catalyst. Green Chem Lett Rev. 3:213–216
Mohebat R, Mojahedi A, Yazdani-Elah-Abadi A (2018) Synthesis of 1,3-Oxazine-4-thione derivatives through an efficient, rapid and green method catalyzed by l-proline in aqueous medium. Org Prep Proced Int 50:424–431
Lukevits E, Demicheva L (1993) Biological activity of furan derivatives. Chem Heterocycl Compd 29:243–267
Vahabinia HR, Karami B, Khodabakhshi S (2013) One-pot synthesis of benzamidonaphtho[2,1-b]furans and benzamidobenzo[b]furans as novel polycyclic heteroaromatic compounds. J Chin Chem Soc 60:1323–1327
Karami B, Khodabakhshi S, Hashemi F (2003) Synthesis of a novel class of benzofurans via a three-component, regiospecific intramolecular heterocylization reaction. Tetrahedron Lett 54:358–3583
Satoh T, Tsuda T, Kushino Y, Miura M, Nomura M (1996) Synthesis of naphthofuran-2(3H)-one derivatives by palladium-catalyzed three-component coupling using naphthols, aldehydes, and carbon monoxide. J Org Chem 61:6476–6477
Yavari I, Anary-Abbasinejad M, Hossaini Z (2003) Reaction between naphthols and dimethyl acetylenedicarboxylate in the presence of phosphites. Synthesis of stable oxa-2λ5-phosphaphenanthrenes, and benzochromene derivatives. Org Biomol Chem 1:560–564
Reza Naimi-Jamal M, Mirzaei M, Bolourtchian M, Sharifi A (2006) One-pot solvent-free preparation of 2-phenyl-1,3,2-aryldioxaborins on acidic alumina. Synth Commun 36:2711–2717
Acknowledgements
Declared none.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, A. Recent development in the synthesis of heterocycles by 2-naphthol-based multicomponent reactions. Mol Divers 25, 1211–1245 (2021). https://doi.org/10.1007/s11030-020-10076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10076-4