Abstract
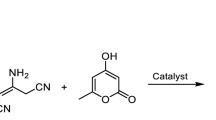
The new multicomponent reaction (MCR) has been found: one-pot selective and efficient formation of the new 5-(4-hydroxy-2-oxo-1,2-dihydropyridin-3-yl)-substituted 5H-chromeno[2,3-b]pyridines in 61–97% yields directly from simple and easily available salicylaldehydes, malononitrile dimer and 4-hydroxypyridine-2(1H)-ones in small amount of pyridine–ethanol catalyst/solvent system. This complex “domino” transformation includes Knoevenagel condensation of salicylaldehyde with malononitrile dimer, Michael addition of 4-hydroxypyridine-2(1H)-one, double Pinner-type reaction cyclization and isomerization with following protonation. This facile multicomponent process opens a new way to 5-(4-hydroxy-2-oxo-1,2-dihydropyridin-3-yl)-substituted 5H-chromeno[2,3-b]pyridine systems, which are promising compounds for the treatment for human inflammatory TNFα-mediated diseases and different biomedical applications.
Graphic abstract





Similar content being viewed by others
References
Schneider P, Schneider G (2017) Privileged structures revisited. Angew Chem Int Ed 56:7971–7994. https://doi.org/10.1002/anie.201702816
Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J (1988) Methods for drug discovery—development of potent, selective, orally effective cholecystokinin antagonist. J Med Chem 31:2235–2246. https://doi.org/10.1021/jm00120a002
Clarke PA, Santos S, Martin WHC (2007) Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem 9:438–440. https://doi.org/10.1039/b700923b
Hayashi Y (2016) Pot economy and one-pot synthesis. Chem Sci 7:866–880. https://doi.org/10.1039/c5sc02913a
Weinstein DS, Gong H, Doweyko AM, Cunningham M, Habte S, Wang JH, Holloway DA, Burke C, Gao L, Guarino V, Carman J, Somerville JE, Shuster D, Salter-Cid L, Dodd JH, Nadler SG, Barrish JC (2011) Azaxanthene based selective glucocorticoid receptor modulators: design, synthesis, and pharmacological evaluation of (S)-4-(5-(1-((1,3,4-thiadiazol-2-yl)amino)-2-methyl1-oxopropan-2-yl)-5H-chromeno[2,3-b]pyridine-2-yl)-2-fluoro-N, N,-dimethylbenzamide (BMS-776532) and its methylene homologue (BMS-791826). J Med Chem 54:7318–7833. https://doi.org/10.1021/jm200879j
Kolokythas G, Pouli N, Marakos P, Pratsinis H, Kletsas D (2006) Design, synthesis and antiproliferative activity of some new azapyranoxanthenone aminoderivatives. Eur J Med Chem 41:71–79. https://doi.org/10.1016/j.ejmech.2005.10.011
Azuine MA, Tokuda H, Takayasu J, Enjyo F, Kapadia GJ (2004) Cancer chemopreventive effect of phenothiazines and related triheterocyclic analogues in the 12-O-tetradecanoylphorbol-13-acetate promoted Epstein–Barr virus early antigen activation and the mouse scin two-stage carcinogenesis models. J Pharmacol Res 49:161–169. https://doi.org/10.1016/j.phrs.2003.07.014
Ukawa K, Ishiguro T, Kuriki H, Nohara A (1985) Studies on antianaphylactic agents. 7. Synthesis of antiallergic 5-oxo-5H-[1]benzopyrano[2,3-b]pyridines. Chem Pharm Bull 33:4432–4437. https://doi.org/10.1021/jm50001a005
Oral EA, Reilly SM, Gomez VA, Meral R, Butz L, Ajluni N, Chenevert TL, Korytnaya E, Neidert AH, Hench R, Rus D, Horowitz JF, Poirier B, Zhao P, Lehmann K, Jain M, Yu R, Liddle C, Ahmadian M, Downes M, Evans RM, Saltiel AR (2017) Inhibition of IKK 3 and TBK1 improves glucose control in subset of patients with type 2 diabetes. Cell Metab 26:157–170. https://doi.org/10.1016/j.cmet.2017.06.006
Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto A, Fujimura A (2008) Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using uman organic anion transporter 3-transfected cells. Eur J Pharmacol 596:166–172. https://doi.org/10.1016/j.ejphar.2008.08.023
Oset-Gasque MJ, Gonzáles MP, Péres-Peña JP, Garcia-Font N, Romero A, del Pino J, Ramos E, Hadjipavlou-Litina D, Soriano E, Chioua M, Samadi A, Raghuvanshi DS, Singh KN, Marco-Contelles J (2014) Toxicological and pharmacological evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines 11-amino-12aryl-8,9,10,12-tetrahydro-7H-chromeno[2,3-b]quinolin-3-ols for the potential prevention and treatment of Alzheimer’s disease. Eur J Med Chem 74:491–501. https://doi.org/10.1016/j.ejmech.2013.12.021
Anderson DR, Hegde S, Reinhard E, Gomez L, Vernier WF, Lee L, Liu S, Sambandam A, Snider PA, Masih L (2005) Aminocyanopyrimidine inhibitors of mutogen activated protein kinase-activated protein kinase 2 (MK2). Bioorg Med Chem Lett 15:1587–1590. https://doi.org/10.1016/j.bmcl.2005.01.067
Michael JP (1997) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 14:11–20. https://doi.org/10.1039/np9971400011
Tatsuta K, Yamaguchi T, Tsuda Y, Yamaguchi Y, Hattori N, Nagai H, Hosokawa S (2007) The first total synthesis and structural determination of YCM1008A. Tetrahedron Lett 48:4187–4190. https://doi.org/10.1016/j.tetlet.2007.04.074
Henning JJ, Gademann K (2010) 4-Hydroxy-2-pyridone alkaloids: structures and synthetic approaches. Nat Prod Rep 27:1168–1185. https://doi.org/10.1039/b911516c
Connolly GP, Duley JA (1999) Uridine and its nucleutides: biological actions, therapeutic potentials. Trends Pharmacol Sci 20:218–225. https://doi.org/10.1016/S0165-6147(99)01298-5
Cardini E, Paladini AC, Caputto R, Leloir LF (1950) Uridine diphosphate glucose: the coenzyme of the galactose-glucose phosphate isomerization. Nature 1950(165):191–192. https://doi.org/10.1038/165191a0
Peters GJ, van Groeningen CJ, Laurensse EJ, Lankelma J, Leyva A, Pinedo HM (1987) Uridine induced hypothermia in mice and rats in relation to plasma and tissue levels of uridine and its metabolites. Cancer Chemother Pharmacol 20:101–108. https://doi.org/10.1007/BF00253962
Connolly GP, Harrison PJ, Stone TW (1993) Action of pyrine and pyrimidine nucleotides on the rat superior cervical ganglion. Br J Pharmacol 110:1297–1304. https://doi.org/10.1111/j.1476-5381.1993.tb13959.x
Elinson MN, Merkulova VM, Ilovaisky AI, Demchuk DV, Belyakov PA, Nikishin GI (2009) Electrochemicaly induced multicomponent assembling of isatins 4-hydroxyquinoline-2(1H)-one and malononitrile: a convenient and efficient way to functionalized [indole-3,4′-pyrano[3,2-c]quinoline] scaffold. Mol Divers 14:833–839. https://doi.org/10.1007/s11030-009-9207z
Vereshchagin AN, Elinson MN, Dorofeeva EO, Stepanov NO, Zaimovskaya TA, Nikishin GI (2013) Electrocatalytic and chemical methods in MHIRC reactions: the first example of the multicomponent assembly of medicinally relevant spirocyclopropyl barbiturates from three different molecules. Tetrahedron 69:1945–1952. https://doi.org/10.1016/j.tet.2012.12.029
Elinson MN, Ilovaisky AI, Merkulova VM, Barba F, Batanero B (2013) General approach to spironaphthylene pentacyclic systems: direct multicomponent assembling of acenaphtenequinone and cyclic carbonyl compounds with two molecules of malononitrile. Tetrahedron 69:7125–7130. https://doi.org/10.1016/j.tet.2013.06.015
Elinson MN, Nasybullin RF, Ryzhkov FV, Egorov MP (2014) Solvent-free and ‘on-water’ multicomponent assembling of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one: A fast and efficient route to the 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene scaffold. C R Chim 17:437–442. https://doi.org/10.1016/j.crci.2013.08.002
Elinson MN, Ryzhkov FV, Korolev VA, Egorov MP (2016) Pot, atom, and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold. Heterocycl Commun 22:11–15. https://doi.org/10.1515/hc-2015-0232
Elinson MN, Ryzhkov FV, Vereshchagin AN, Korshunov DA, Novikov RA, Egorov MP (2017) ‘On-solvent’ new domino reaction of salicylaldehyde, malononitrile and 4-hydroxy-6-methylpyridin-2(1H)-one: fast and efficient approach to medicinally relevant 4-pyridinyl-2-amino-4H-chromene scaffold. Mendeleev Commun 27:559–561. https://doi.org/10.1016/j.mencom.2017.11.006
Vereshchagin AN, Elinson MN, Anisina YE, Ryzhkov FV, Goloveshkin AS, Novikov RA, Egorov MP (2017) Synthesis, structural, spectroscopic and docking studies of new 5-C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J Mol Struct 1146:766–772. https://doi.org/10.1016/j.molstruc.2017.06.044
Elinson MN, Vereshchagin AN, Anisina YE, Goloveshkin AS, Ushakov IE, Egorov MP (2018) PASE facile and efficient multicomponent approach to the new type of 5-C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun 28:372–374. https://doi.org/10.1016/j.mencom.2018.07.010
Sheldon RA, Arends IWCE, Hanefeld U (2007) Green chemistry and catalysis. Wiley-VHC, Weinheim
Martins MAP, Frizzo CP, Moreira DN, Buriol L, Machado P (2009) Solvent-free heterocyclic synthesis. Chem Rev 109:4140–4182. https://doi.org/10.1021/cr9001098
Elinson MN, Medvedev MG, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2013) Solvent-free cascade assembling of salicylic aldehydes and malononitrile: rapid and efficient approach to 2-amino-4H-chromene scaffold. Mendeleev Commun 23:94–95. https://doi.org/10.1016/j.mencom.2013.03.014
Butler RN, Coyne AG (2016) Organic synthesis reactions on-water at the organic-liquid water interface. Org Biomol Chem 14:9945–9960. https://doi.org/10.1039/c6ob01724j
Demchuk DV, Elinson MN, Nikishin GI (2011) ‘On-water’ Knoevenagel condensation of isatins with malononitrile. Mendeleev Commun 21:224–225. https://doi.org/10.1016/j.mencom.2011.07.018
Elinson MN, Vereshchagin AN, Anisina YE, Krymov SK, Fakhrutdinov AM, Egorov MP (2019) Selective multicomponent ‘one-pot’ approach to the new 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-chromeno[2,3-b]pyridine scaffold in pyridine-ethanol catalyst/solvent system. Monatsh Chem. https://doi.org/10.1007/s00706-019-02388-5
Elinson MN, Merkulova VM, Ilovaisky AI, Demchuk DV, Belyakov PA, Nikishin GI (2010) Cascade assembly of N, N′-dialkylbarbituric acids and aldehydes: a simple and efficient one pot approach to the substituted 1,5-dihydro-2,2′-H-spiro(furo[2,3-d]pyrimidine)-2,2,4,4′,6(1′H,3H,3H′)-pentone framework. Tetrahedron Lett 51:6598–6601. https://doi.org/10.1016/10.1016/j.tetlet.2010.10.041
Mittelbach M (1985) An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Monatsh Chem 116:689–691. https://doi.org/10.1007/BF00798796
Kraus GA, Wanninayaki UK, Bottoms J (2016) Triacetic acid lactone as common intermediate for the synthesis of 4-hydroxy-2-pyridones and 4-amino-2-pyrones. Tetrahedron Lett 57:1293–1295. https://doi.org/10.1016/j.tetlet.2016.02.043
Acknowledgements
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project 18-03-00212).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elinson, M.N., Vereshchagin, A.N., Anisina, Y.E. et al. Pot, atom and step economic (PASE) assembly of salicylaldehydes, malononitrile dimer and 4-hydroxypyridine-2(1H)-ones into medicinally relevant 5H-chromeno[2,3-b]pyridine scaffold. Mol Divers 24, 617–626 (2020). https://doi.org/10.1007/s11030-019-09968-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09968-x