Abstract
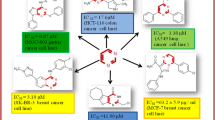
Pyran-4-one (maltol, kojic acid and chlorokojic acid 1) esters of adamantan-1-ylacetic acid were prepared through efficient synthetic routes in good yields and evaluated for their in vitro antiproliferative activity on four cancer cell lines: K562 (chronic myelogenous leukemia), HeLa (cervical cancer), Caco-2 (colorectal adenocarcinoma) and NCI-H358 (bronchioalveolar carcinoma). The results indicate that the presence and the position of the adamantyl acyl group or chlorine atom are the necessary requirement for antitumor activity of pyranone systems. Derivatives of kojic acid with either free (compounds 1 and 8) or acylated 5-OH group (compounds 2 and 9) have shown good-to-moderate activity (IC50 values ranging from 13.1 to 43.0 μM) on all cell lines. Adamantyl kojic acid derivative 5 with a free OH group on the position 2 showed activity only on the K562 cell line. It seems that removal of halogen or adamantyl unit from position 2 elicits antileukemic activity, as observed in compound 5. The positive influence of the adamantyl unit was also observed on a 3-OH acylated derivative of maltol I which was also selectively active on the same cell line. 5-O-benzylated adamantyl compounds 6 and 7 and unmodified starting pyranones were found to be inactive. Antibacterial activity of compounds was also evaluated on S. aureus ATCC 13709, M. catarrhalis ATCC 23246, E. faecalis ATCC29212 and E. coli TolC-Tn10, but no activity was observed (MIC values 128–256 µg/mL).
Graphical abstract





Similar content being viewed by others
References
Brtko J, Rondahl L, Ficková M, Hudecová D, Eybl V, Uher M (2004) Kojic acid and its derivatives: history and present state of art. Cent Eur J Public Health 12:S16–S18
Gonçalez ML, Marcussi DG, Fioramonti Calixto GM, Corrêa MA, Chorilli M (2015) Structural characterization and in vitro antioxidant activity of kojic dipalmitate loaded W/O/W multiple emulsions intended for skin disorders. Biomed Res Int 2015:1–8. https://doi.org/10.1155/2015/304591
Piršelová K, Baláž Š, Ujhelyová R, Šturdík E, Veverka M, Uher M, Brtko J (1996) Quantitative structure-time-activity relationships (QSTAR): growth inhibition of Escherichia coli by nonionizable kojic acid derivatives. Quant Struct-Act Relat 15:87–93. https://doi.org/10.1002/qsar.19960150202
Emami S, Ghafouri E, Faramarzi MA, Samadi N, Irannejad H, Foroumadi A (2013) Mannich bases of 7-piperazinylquinolones and kojic acid derivatives: synthesis, in vitro antibacterial activity and in silico study. Eur J Med Chem 68:185–191. https://doi.org/10.1016/j.ejmech.2013.07.032
Bransová J, Brtko J, Uher M, Novotný L (1995) Antileukemic activity of 4-pyranone derivatives. Int J Biochem Cell Biol 27:701–706. https://doi.org/10.1016/1357-2725(95)00031-J
Yoo DS, Lee J, Choi SS, Rho HS, Cho DH, Shin WC, Cho JY (2010) A modulatory effect of novel kojic acid derivatives on cancer cell proliferation and macrophage activation. Pharmazie 65:261–266. https://doi.org/10.1691/ph.2010.9764
Fickova M, Pravdova E, Rondhal L, Uher M, Brtko J (2008) In vitro antiproliferative and cytotoxic activities of novel kojic acid derivatives: 5-benzyloxy-2-selenocyanatomethyl - and 5-methoxy-2-selenocyanatomethyl-4-pyranone. J Appl Toxicol 28:554–559. https://doi.org/10.1002/jat.1300
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S, Li C (2014) Ciprofloxacin containing Mannich base and its copper complex induce antitumor activity via different mechanism of action. Int J Oncol 45:2092–2100. https://doi.org/10.3892/ijo.2014.2611
Aytemir MD, Septioğlu E, Çalış Ü (2010) Synthesis and anticonvulsant activity of new kojic acid derivatives. Arzneimittelforschung 60(1):22–29. https://doi.org/10.1055/s-0031-1296244
Rho HS, Goh M, Lee J, Ahn SM, Yeon J, Yoo DS, Kim DH, Kim HG, Cho JY (2011) Ester derivatives of kojic acid and polyphenols containing adamantane moiety with tyrosinase inhibitory and anti-inflammatory properties. Bull Korean Chem Soc 32:1411–1414. https://doi.org/10.5012/bkcs.2011.32.4.1411
Yellamma K, Jyothi P (2017) In silico approach for validation of maltol derivatives as acetylcholinesterase inhibitors. Int J Pharm Sci Rev Res 42(1):300–306
Li W, Su X, Han Y, Xu Q, Zhang J, Wang Z, Wang Y (2015) Maltol, a Maillard reaction product, exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. RSC Adv 5:101850–101859. https://doi.org/10.1039/C5RA17960B
Aytemir MD, Karakaya G (2012) Kojic acid derivatives. In: Ekinci D (ed) Medicinal chemistry and drug design. InTechOpen, pp 1–26. https://doi.org/10.5772/2457
Zirak M, Eftekhari-Sis B (2015) Kojic acid in organic synthesis. Turk J Chem 39:439–496. https://doi.org/10.3906/kim-1502-55
Liu J, Obando D, Liao V, Lifa T, Codd R (2011) The many faces of the adamantyl group in drug design. Eur J Med Chem 46:1949–1963. https://doi.org/10.1016/j.ejmech.2011.01.047
Lamoureux G, Artavia G (2010) Use of the adamantane structure in medicinal chemistry. Curr Med Chem 17:2967–2978. https://doi.org/10.2174/092986710792065027
Petrović Peroković V, Car Ž, Opačak-Bernardi T, Martin-Kleiner I, Kralj M, Tomić S (2017) In vitro antiproliferative study of novel adamantyl pyridin-4-ones. Mol Divers 21:881–891. https://doi.org/10.1007/s11030-017-9763-6
Choi YJ, Rho HS, Baek HS, Hong YD, Joo YH, Shin SS, Kim JM (2014) Synthesis and biological evaluation of kojic acid derivatives as tyrosinase inhibitors. Bull Korean Chem Soc 35(12):3647–3650. https://doi.org/10.5012/bkcs.2014.35.12.3647
Car Ž, Hrenar T, Petrović Peroković V, Ribić R, Seničar M, Tomić S (2014) Mannosylated N-aryl substituted 3-hydroxypyridine-4-ones: synthesis, hemagglutination inhibitory properties and molecular modeling. Chem Biol Drug Des 84:393–401. https://doi.org/10.1111/cbdd.12329
Petrović Peroković V, Ribić R, Car Ž, Tomić S (2016) Comparison of inhibitory activities of meta and para substituted N-aryl 3-hydroxypyridin-4-one mannosides towards type 1 fimbriated E. coli. Croat Chem Acta 89(2):237–242. https://doi.org/10.5562/cca2890
Aytemir MD, Özçelik B (2010) A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur J Med Chem 45:4089–4095. https://doi.org/10.1016/j.ejmech.2010.05.069
Aerts J, Vandenbroucke RE, Dera R, Balusu S, Van Wonterghem E, Moons L, Libert C, Dehaen W, Arckens L (2015) Synthesis and validation of a hydroxypyrone-based, potent, and specific matrix metalloproteinase-12 inhibitor with anti-inflammatory activity in vitro and in vivo. Mediat Inflamm 2015:1–9. https://doi.org/10.1155/2015/510679
Aytemir MD, Özçelik B (2011) Synthesis and biological activities of new Mannich bases of chlorokojic acid derivatives. Med Chem Res 20:443–452. https://doi.org/10.1007/s00044-010-9338-x
Borah R, Talukdar D, Gorai S, Bain D, Manna D (2014) Bilayer interaction and protein kinase C–C1 domain binding studies of kojic acid esters. RSC Adv 4:25520–25531. https://doi.org/10.1039/c4ra02352h
Sarkar TK, Ghosh SK, Subba Rao PSV, Satapathi TK, Mamdapur VR (1992) Cyclopentanoid allylsilanes in synthesis of di- and triquinanes. A stereoselective synthesis of (±)-hirsutene. Tetrahedron 48(33):6897–6908. https://doi.org/10.1016/s0040-4020(01)89880-x
Campbell AL, Miyano M (1989) Kojic acid ether-ester derivatives. US Patent Number 4,812,474
Jay JM, Rivers GM (1984) Antimicrobial activity of some food flavoring compounds. J Food Saf 6(2):129–139. https://doi.org/10.1111/j.1745-4565.1984.tb00609.x
Karakaya G, Aytemir MD, Özçelik B, Çalış Ü (2013) Design, synthesis and in vivo/in vitro screening of novel chlorokojic acid derivatives. J Enzyme Inhib Med Chem 28(3):627–638. https://doi.org/10.3109/14756366.2012.666538
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1(4):337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Virtual Computational Chemistry Laboratory. http://www.vcclab.org/lab/alogps/. Accessed 6 Feb 2019
Mickisch G, Fajta S, Keilhauer G, Schlick E, Tschada R, Alken P (1990) Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT). Urol Res 18:131–136
Supek F, Šumanovac Ramljak T, Marjanović M, Buljubašić M, Kragol G, Ilić N, Šmuc T, Zahradka D, Mlinarić-Majerski K, Kralj M (2011) Could log P be a principal determinant of biological activity in 18-crown-6 ethers? Synthesis of biologically active adamantane-substituted diaza-crowns. Eur J Med Chem 46:3444–3544. https://doi.org/10.1016/j.ejmech.2011.05.009
Acknowledgements
We wish to thank kindly Dr Hana Čipčić Paljetak and Dr Donatella Verbanac from the School of Medicine, University of Zagreb, for investigating the antibacterial activity of compounds. We would also like to thank the Croatian Science Foundation for support of this work (Project IP-2014-09-7899).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peroković, V.P., Car, Ž., Usenik, A. et al. Adamantyl pyran-4-one derivatives and their in vitro antiproliferative activity. Mol Divers 24, 253–263 (2020). https://doi.org/10.1007/s11030-019-09948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09948-1