Abstract
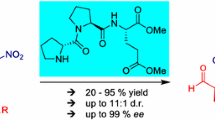
A combined organocatalytic and multicomponent synthetic approach was designed for the preparation of selenium-based peptoids and peptide–peptoid conjugates. This single-step synthetic protocol comprises the organocatalytic asymmetric insertion of phenylselenium in the aldehyde moiety followed by the Ugi four-component reaction which results in obtaining the desired compounds in good-to-moderate yields and with good-to-excellent levels of stereoselectivity.
Graphical abstract


Similar content being viewed by others
References
Wirth T (2012) Organoselenium chemistry: synthesis and reactions. Wiley-VCH & CO., Weinheim
Freudendahl DM, Shahzad SA, Wirth T (2009) Recent advances in organoselenium chemistry. Eur J Org Chem. https://doi.org/10.1002/ejoc.200801171
Nicolaou KC, Petasis NA (1984) Selenium in natural products synthesis. CIS, Inc., Pennsylvania. https://doi.org/10.1002/ange.19850970448
Sharpless KB, Lauer RF, Teranishi AY (1973) Electrophilic and nucleophilic organoselenium reagents. New routes to. alpha., beta.-unsaturated carbonyl compounds. J Am Chem Soc 95:6137–6139. https://doi.org/10.1021/ja00799a062
Godoi M, Paixão MW, Braga AL (2011) Chiral organoselenium-transition-metal catalysts in asymmetric transformations. Dalton Trans 40:11347–11355. https://doi.org/10.1039/C1DT11022E
Braga AL, Lüdtke DS, Vargas F, Braga RC (2006) Catalytic applications of chiral organoselenium compounds in asymmetric synthesis. Synlett 10:1453–1466. https://doi.org/10.1055/s-2006-941592
Braga AL, Lüdtke DS, Vargas F (2006) Enantioselective synthesis mediated by catalytic chiral organoselenium compounds. Curr Org Chem 10:1921–1938. https://doi.org/10.2174/138527206778521204
Frizon TE, Rampon DS, Gallardo H, Merlo AA, Schneider PH, Rodrigues OED, Braga AL (2012) Selenides and diselenides containing oxadiazoles: a new class of functionalised materials. Liq Cryst 39:769–777. https://doi.org/10.1080/02678292.2012.680505
Frizon TE, Rafique J, Saba S, Bechtold IH, Gallardo H, Braga AL (2015) Synthesis of functionalized organoselenium materials: selenides and diselenides containing cholesterol. Eur J Org Chem. https://doi.org/10.1002/ejoc.201500124
Reich HJ, Hondal RJ (2016) Why nature chose selenium. ACS Chem Biol 11:821–841. https://doi.org/10.1021/acschembio.6b00031
Santi C, Tidei C, Scalera C, Piroddi M, Galli F (2013) Selenium containing compounds from poison to drug candidates: a review on the GPx-like activity. Curr Chem Biol 7:25–36. https://doi.org/10.2174/2212796811307010003
Mugesh G, du Mont W-W, Sies H (2001) Chemistry of biologically important synthetic organoselenium compounds. Chem Rev 101:2125–2180. https://doi.org/10.1021/cr000426w
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443. https://doi.org/10.1126/science.1083516
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777. https://doi.org/10.1152/physrev.00039.2013
Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F (1991) Selenocysteine: the 21st amino acid. Mol Microbiol 5:515–520. https://doi.org/10.1111/j.1365-2958.1991.tb00722.x
Atkins JF, Gesteland RF (2000) The twenty-first amino acid. Nature 407:463–464. https://doi.org/10.1038/35035189
Wessjohann LA, Schneider A (2008) Synthesis of selenocysteine and its derivatives with an emphasis on selenenylsulfide (-Se-S-) formation. Chem Biodivers 5:375–388. https://doi.org/10.1002/cbdv.200890038
Low SC, Berry MJ (1996) Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem Sci 21:203–208. https://doi.org/10.1016/S0968-0004(96)80016-8
Donovan J, Copeland PR (2010) Threading the needle: getting selenocysteine into proteins. Antioxid Redox Signal 12:881–892. https://doi.org/10.1089/ars.2009.2878
Turanov AA, Xu XM, Carlson BA, Yoo MH, Gladyshev VN, Hatfield DL (2011) Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis. Adv Nutr 2:122–128. https://doi.org/10.3945/an.110.000265
Wessjohann LA, Schneider A, Abbas M, Brandt W (2007) Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem 388:997–1006. https://doi.org/10.1515/BC.2007.138
Jacob C, Giles GI, Giles NM, Sies H (2003) Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed 42:4742–4758. https://doi.org/10.1002/anie.200300573
Iwaoka M, Arai K (2013) From sulfur to selenium. A new research arena in chemical biology and biological chemistry. Curr Chem Biol 7:2–24. https://doi.org/10.2174/2212796811307010002
Andreadou I, Menge WMPB, Commandeur JNM, Worthington EA, Vermeulen PE (1996) Synthesis of novel se-substituted selenocysteine derivatives as potential kidney selective prodrugs of biologically active selenol compounds: evaluation of kinetics of β-elimination reactions in rat renal cytosol. J Med Chem 39:2040–2046. https://doi.org/10.1021/jm950750x
Stocking EM, Schwarz JN, Senn H, Salzmann M, Silks LA (1997) Synthesis of L-selenocystine, L-[77Se]selenocystine and L-tellurocystine. J Chem Soc Perkin Trans 1:2443–2447. https://doi.org/10.1039/A600180G
Schneider A, Rodrigues OED, Paixão MW, Appelt HR, Braga AL, Wessjohann LA (2006) Stereoselective synthesis of Boc-protected l-seleno- and tellurolanthionine, l-seleno- and tellurocystine and derivatives. Tetrahedron Lett 47:1019–1021. https://doi.org/10.1016/j.tetlet.2005.11.101
Braga AL, Wessjohann LA, Taube PS, Galetto FZ, Molinos FA (2010) Straightforward method for the synthesis of selenocysteine and selenocystine derivatives from l-serine methyl ester. Synthesis 18:3131–3137. https://doi.org/10.1055/s-0030-1258188
Shimodaira S, Iwaoka M (2017) Improved synthetic routes to the selenocysteine derivatives useful for Boc-based peptide synthesis with benzylic protection on the selenium atom. Arkivok. https://doi.org/10.3998/ark.5550190.p009.803
Muttenthaler M, Alewood PF (2008) Selenopeptide chemistry. J Pept Sci 14:1223–1239. https://doi.org/10.1002/psc.1075
Besse D, Moroder L (1997) Synthesis of selenocysteine peptides and their oxidation to diselenide-bridged compounds. J Pept Sci 3:442–453. https://doi.org/10.1002/(SICI)1099-1387(199711)3:6%3c442:AID-PSC122%3e3.0.CO;2-2
Okeley NM, Zhu Y, van der Donk WA (2000) Facile chemoselective synthesis of dehydroalanine-containing peptides. Org Lett 2:3603–3606. https://doi.org/10.1021/ol006485d
Gieselman MD, Xie L, van der Donk WA (2001) Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org Lett 3:1331–1334. https://doi.org/10.1021/ol015712o
Braga AL, Lüdtke DS, Paixão MW, Alberto EE, Stefani HA, Juliano L (2005) Straightforward synthesis of non-natural selenium containing amino acid derivatives and peptides. Eur J Org Chem. https://doi.org/10.1002/ejoc.200500530
Flemer S Jr, Lacey BM, Hondal RJ (2008) Synthesis of peptide substrates for mammalian thioredoxin reductase. J Pept Sci 14:637–647. https://doi.org/10.1002/psc.961
Abbas M, Bethke J, Wessjohann LA (2006) One pot synthesis of selenocysteine containing peptoid libraries by Ugi multicomponent reactions in water. Chem Commun. https://doi.org/10.1039/B514597J
Mecklenburg S, Shaaban S, Ba LA, Burkholz T, Schneider T, Diesel B, Kiemer AK, Röseler A, Becker K, Reichrath J, Stark A, Tilgen W, Abbas M, Wessjohann LA, Sasse F, Jacob C (2009) Exploring synthetic avenues for the effective synthesis of selenium- and tellurium-containing multifunctional redox agents. Org Biomol Chem 7:4753–4762. https://doi.org/10.1039/B907831B
Alberto EE, Soares LC, Sudati JH, Borges ACA, Rocha JBT, Braga AL (2009) Efficient synthesis of modular amino acid derivatives containing selenium with pronounced GPx-like activity. Eur J Org Chem 25:4211–4214. https://doi.org/10.1002/ejoc.200900485
Takei T, Urabe Y, Asahina Y, Hojo H, Nomura T, Dedachi K, Arai K, Iwaoka M (2014) Model study using designed selenopeptides on the importance of the catalytic triad for the antioxidative functions of glutathione peroxidase. J Phys Chem B 118:492–500. https://doi.org/10.1021/jp4113975
Iawoka M (2013) Biochalcogen chemistry: the biological chemistry of sulfur, selenium, and tellurium. ACS symposium series. American Chemical Society, Washington, pp 163–177. https://doi.org/10.1021/bk-2013-1152.ch008
Abbas M, Wessjohann LA (2012) Direct synthesis of sensitive selenocysteine peptides by the Ugi reaction. Org Biomol Chem 10:9330–9333. https://doi.org/10.1039/C2OB26552D
Kaluđerović GN, Abbas M, Kautz HC, Wadaam MAM, Lennicke C, Seliger B, Wessjohann LA (2017) Methionine and seleno-methionine type peptide and peptoid building blocks synthesized by five-component five-center reactions. Chem Commun 53:3777–3780. https://doi.org/10.1039/C7CC00399D
Liu H, Dömling A (2009) One-pot synthesis of highly functionalized seleno amino acid derivatives. Chem Biol Drug Des 74:302–308. https://doi.org/10.1111/j.1747-0285.2009.00854.x
Ramón DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed 44:1602–1634. https://doi.org/10.1002/anie.200460548
Guillena G, Ramón DJ, Yus M (2007) Organocatalytic enantioselective multicomponent reactions (OEMCRs). Tetrahedron Asymmetry 18:693–700. https://doi.org/10.1016/j.tetasy.2007.03.002
de Graaff C, Ruijter E, Orru RVA (2012) Recent developments in asymmetric multicomponent reactions. Chem Soc Rev 41:3969–4009. https://doi.org/10.1039/C2CS15361K
Marson CM (2012) Multicomponent and sequential organocatalytic reactions: diversity with atom-economy and enantiocontrol. Chem Soc Rev 41:7712–7722. https://doi.org/10.1039/C2CS35183H
Cioc RC, Ruijter E, Orru RVA (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16:2958–2975. https://doi.org/10.1039/C4GC00013G
Tietze LF, Modi A (2000) Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med Res Rev 3:4–322. https://doi.org/10.1002/1098-1128(200007)20:4%3c304:AID-MED3%3e3.0.CO;2-8
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. https://doi.org/10.1021/cr0505728
Kalinski C, Lemoine H, Schmidt J, Burdack C, Kolb J, Umkehrer M, Ross G (2008) Multicomponent reactions as a powerful tool for generic drug synthesis. Synthesis 24:4007–4011. https://doi.org/10.1055/s-0028-1083239
Shabaan S, Ba LA, Abbas M, Burkholz T, Denkert A, Gohr A, Wessjohann LA, Sasse F, Weber W, Jacob C (2009) Multicomponent reactions for the synthesis of multifunctional agents with activity against cancer cells. Chem Commun 21:4702–4704. https://doi.org/10.1039/B823149D
Zarganes-Tzitzikas T, Dömling A (2014) Modern multicomponent reactions for better drug syntheses. Org Chem Front 1:834–837. https://doi.org/10.1039/C4QO00088A
Nascimento V, Alberto EE, Tondo DW, Dambrowski D, Detty MR, Nome F, Braga AL (2012) GPx-like activity of selenides and selenoxides: experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J Am Chem Soc 134:138–141. https://doi.org/10.1021/ja209570y
Soares LC, Alberto EE, Schwab RS, Taube OS, Nascimento V, Rodrigues OED, Braga AL (2012) Ephedrine-based diselenide: a promiscuous catalyst suitable to mimic the enzyme glutathione peroxidase (GPx) and to promote enantioselective C–C coupling reactions. Org Biomol Chem 10:6595–6599. https://doi.org/10.1039/C2OB25539A
Rafique J, Canto RFS, Saba S, Barbosa FAR, Braga AL (2016) Recent advances in the synthesis of biologically relevant selenium-containing 5-membered heterocycles. Curr Org Chem 20:166–188. https://doi.org/10.2174/1385272819666150810222057
Barbosa FAR, Canto RFS, Saba S, Rafique J, Braga AL (2016) Synthesis and evaluation of dihydropyrimidinone-derived selenoesters as multi-targeted directed compounds against Alzheimer’s disease. Bioorg Med Chem 24:5762–5770. https://doi.org/10.1016/j.bmc.2016.09.031
Echemendía R, de la Torre AF, Monteiro JL, Pila M, Corrêa AG, Westermann B, Rivera DG, Paixão MW (2015) Highly stereoselective synthesis of natural-product-like hybrids by an organocatalytic/multicomponent reaction sequence. Angew Chem Int Ed 54:7621–7625. https://doi.org/10.1002/anie.201412074
de La Torre AF, Rivera DG, Concepcion O, Echemendía R, Corrêa AG, Paixão MW (2016) Multicomponent synthesis of cyclic depsipeptide mimics by Ugi reaction including cyclic hemiacetals derived from asymmetric organocatalysis. J Org Chem 81:803–809. https://doi.org/10.1021/acs.joc.5b02158
Deobald AM, Corrêa AG, Rivera DG, Paixão MW (2012) Organocatalytic asymmetric epoxidation and tandem epoxidation/Passerini reaction under eco-friendly reaction conditions. Org Biomol Chem 10:7681–7686. https://doi.org/10.1039/C2OB26247A
de La Torre AF, Rivera DG, Ferreira MAB, Corrêa AG, Paixão MW (2013) Multicomponent combinatorial development and conformational analysis of prolyl peptide–peptoid hybrid catalysts: application in the direct asymmetric Michael addition. J Org Chem 78:10221–10232. https://doi.org/10.1021/jo401609z
Tiecco M, Carlone A, Sternativo S, Marini F, Bartoli G, Melchiorre P (2007) Organocatalytic asymmetric α-selenenylation of aldehydes. Angew Chem Int Ed 46:6882–6885. https://doi.org/10.1002/anie.200702318
Marigo M, Wabnitz TC, Fielenbach D, Jørgensen KA (2005) Enantioselective organocatalyzed α sulfenylation of aldehydes. Angew Chem Int Ed 44:794–797. https://doi.org/10.1002/anie.200462101
Franzen J, Marigo M, Fielenbach D, Wabnitz TC, Kjarsgaard A, Jørgensen KA (2005) A general organocatalyst for direct α-functionalization of aldehydes: stereoselective C–C, C–N, C–F, C–BR, and C–S bond-forming reactions. Scope and mechanistic insights. J Am Chem Soc 127:18296–18304. https://doi.org/10.1021/ja056120u
Armarego WLF (2017) Purification of laboratory chemicals, 8th edn. Butterworth Heinemann, Oxford, pp 95–634
Acknowledgements
A.F. de la Torre is grateful to financial support from CNPq (14/403846) and Fondecyt 3170003. We also gratefully acknowledge financial support from CNPq (INCT-Catálise), CAPES (CAPES-MES/Cuba Program) and FAPESP (14/50249-8 and 15/17141-1). A. Ali is thankful to TWAS-CNPq for the fellowship and support from the higher education commission of Pakistan (Project No. 21-2037/SRGP/R&D/HEC/2018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de la Torre, A.F., Ali, A., Galetto, F.Z. et al. One-pot organocatalytic/multicomponent approach for the preparation of novel enantioenriched non-natural selenium-based peptoids and peptide–peptoid conjugates. Mol Divers 24, 1–10 (2020). https://doi.org/10.1007/s11030-019-09923-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09923-w