Abstract
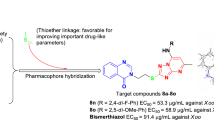
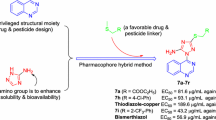
A total of eighteen 2-((2-(4-(1H-1,2,4-triazol-1-yl)phenyl)quinazolin-4-yl)oxy)-N-phenylacetamide derivatives were designed and synthesized, via hybrid pharmacophore approach. Among these compounds, chemical structure of compound 4a was unambiguously confirmed by means of single-crystal X-ray diffraction analysis. All the compounds were evaluated in vitro for their inhibition activity against several important phytopathogenic bacteria and fungi in agriculture. The obtained results indicated that several compounds demonstrated potent antibacterial activity against Xanthomonas oryzae pv. oryzae (Xoo). For example, compounds 4c, 4g and 4q had EC50 values of 35.0, 36.5 and 32.4 µg/mL toward this bacterium, respectively, around 1.5 times more active than commercial bactericide bismerthiazol (EC50 = 89.8 µg/mL). Additionally, compounds 4j and 4p were found to display comparable antifungal activity against Gloeosporium fructigenum at 50 µg/mL, to commercial fungicide hymexazol. Finally, the relationships between antibacterial activities and molecular structures of this class of compounds were discussed in detail.
Graphical abstract





Similar content being viewed by others
References
Jia CY, Xu LY, Yu X, Ding YB, Jin B, Zhang MZ, Zhang WH, Yang GF (2018) An efficient synthesis and antifungal evaluation of natural product streptochlorin and its analogues. Fitoterapia 125:106–110. https://doi.org/10.1016/j.fitote.2017.12.017
Ahmad M, Aga MA, Bhat JA, Kumar B, Rouf A, Capalash N, Mintoo MJ, Kumar A, Mahajan P, Mondhe DM, Nargotra A, Sharma PR, Zargar MA, Vishwakarma RA, Shah BA, Taneja SC, Hamid A (2017) Exploring derivatives of quinazoline alkaloid L-vasicine as cap groups in the design and biological mechanistic evaluation of novel antitumor histone deacetylase inhibitors. J Med Chem 60:3484–3497. https://doi.org/10.1021/acs.jmedchem.7b00322
Van Horn KS, Burda WN, Fleeman R, Shaw LN, Manetsch R (2014) Antibacterial activity of a series of N 2, N 4-disubstituted quinazoline-2,4-diamines. J Med Chem 57:3075–3093. https://doi.org/10.1021/jm500039e
Bedi PMS, Kumar V, Mahajan MP (2004) Synthesis and biological activity of novel antibacterial quinazolines. Bioorg Med Chem Lett 14:5211–5213. https://doi.org/10.1016/j.bmcl.2004.07.065
Berest GG, Voskoboynik OY, Kovalenko SI, Antypenko OM, Nosulenko IS, Katsev AM, Shandrovskaya OS (2011) Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides. Eur J Med Chem 46:6066–6074. https://doi.org/10.1016/j.ejmech.2011.10.022
Liu F, Huang YJ (2011) Antifungal bioactivity of 6-bromo-4-ethoxyethylthio quinazoline. Pestic Biochem Phys 101:248–255. https://doi.org/10.1016/j.pestbp.2011.10.002
Luo H, Liu JJ, Jin LH, Hu DY, Chen Z, Yang S, Wu J, Song BA (2013) Synthesis and antiviral bioactivity of novel (1E,4E)-1-aryl-5-(2-(quinazolin-4-yloxy) phenyl)-1,4-pentadien-3-one derivatives. Eur J Med Chem 63:662–669. https://doi.org/10.1016/j.ejmech.2013.02.035
Sharma A, Luxami V, Paul K (2013) Synthesis, single crystal and antitumor activities of benzimidazole-quinazoline hybrids. Bioorg Med Chem Lett 23:3288–3294. https://doi.org/10.1016/j.bmcl.2013.03.107
Niño-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7:303–324. https://doi.org/10.1111/j.1364-3703.2006.00344.x
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320. https://doi.org/10.1007/s001220050565
Wang PY, Zhou L, Zhou J, Wu ZB, Xue W, Song BA, Yang S (2016) Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg Med Chem Lett 26:1214–1217. https://doi.org/10.1016/j.bmcl.2016.01.029
Kulabas N, Tatar E, Özakpinar ÖB, Özsavci D, Pannecouque C, De Clercq E, Kücükgüzel I (2016) Synthesis and antiproliferative evaluation of novel 2-(4H-1,2,4-triazole-3-ylthio)acetamide derivatives as inducers of apoptosis in cancer cells. Eur J Med Chem 121:58–70. https://doi.org/10.1016/j.ejmech.2016.05.017
Cavusoglu BK, Yurttas L, Cankilic MY, Kaplancikli ZA (2017) Synthesis, antibacterial, antifungal, antimycobacterial activity evaluation of novel 1,2,4-triazole derivatives bearing 4-aminophenyl moiety. Lett Drug Des Discov 14:938–948. https://doi.org/10.2174/1570180814666161130103624
Pan DW, Du H, Lv XY, Bao XP (2016) Synthesis and antibacterial activities of novel quinazoline-2,4-dione derivatives containing the 1,2,4-triazole Schiff-base unit. Chin J Org Chem 36:818–825. https://doi.org/10.6023/cjoc201510005
Zhang TY, Li C, Li YR, Li XZ, Sun LP, Zheng CJ, Piao HR (2016) Synthesis and antimicrobial evaluation of aminoguanidine and 3-amino-1,2,4-triazole derivatives as potential antibacterial agents. Lett Drug Des Discov 13:1063–1075. https://doi.org/10.2174/1570180813666160819151239
Lin GS, Duan WG, Yang LX, Huang M, Lei FH (2017) Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules 22:193. https://doi.org/10.3390/molecules22020193
Boraei ATA, Gomaa MS, El Ashry ESH, Duerkop A (2017) Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur J Med Chem 125:360–371. https://doi.org/10.1016/j.ejmech.2016.09.046
Ma L, Xiao Y, Li C, Xie ZL, Li DD, Wang YT, Ma HT, Zhu HL, Wang MH, Ye YH (2013) Synthesis and antioxidant activity of novel Mannich base of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan. Bioorg Med Chem 21:6763–6770. https://doi.org/10.1016/j.bmc.2013.08.002
Havrylyuk D, Roman O, Lesyk R (2016) Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline-thiazolidine-based hybrids. Eur J Med Chem 113:145–166. https://doi.org/10.1016/j.ejmech.2016.02.030
Yang L, Ge SJ, Huang J, Bao XP (2018) Synthesis of novel (E)-2-(4-(1H-1,2,4-triazol-1-yl)styryl)-4-(alkyl/arylmethyleneoxy)quinazoline derivatives as antimicrobial agents. Mol Diver 22:71–82. https://doi.org/10.1007/s11030-017-9792-1
Singh M, Singh SK, Gangwar M, Nath G, Singh SK (2014) Design, synthesis and mode of action of some benzothiazole derivatives bearing an amide moiety as antibacterial agents. RSC Adv 4:19013–19023. https://doi.org/10.1039/c4ra02649g
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl)acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Adv 7:34005–34011. https://doi.org/10.1039/c7ra04819j
Fan ZJ, Shi J, Bao XP (2018) Synthesis and antimicrobial evaluation of novel 1,2,4-triazole thioether derivatives bearing a quinazoline moiety. Mol Diver 22:657–667. https://doi.org/10.1007/s11030-018-9821-8
Liu JH, Liu Y, Jian JY, Bao XP (2013) Synthesis and fungicidal activities of novel quinazoline derivatives containing 1,2,4-Triazole Schiff-base unit. Chin J Org Chem 33:370–374. https://doi.org/10.6023/cjoc201209023
Jadhav GR, Shaikh MU, Kale RP, Shiradkar MR, Gill CH (2009) SAR study of clubbed [1,2,4]-triazolyl with fluorobenzimidazoles as antimicrobial and antituberculosis agents. Eur J Med Chem 44:2930–2935. https://doi.org/10.1016/j.ejmech.2008.12.001
Wang X, Li P, Li ZN, Yin J, He M, Xue W, Chen ZW, Song BA (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. https://doi.org/10.1021/jf403193q
Zhao M, Dai ZC, Qian SS, Liu JY, Xiao Y, Lu AM, Zhu HL, Wang JX, Ye YH (2014) Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J Agric Food Chem 62:9637–9643. https://doi.org/10.1021/jf504359p
Xiong L, Li H, Jiang LN, Ge JM, Yang WC, Zhu XL, Yang GF (2017) Structure-based discovery of potential fungicides as succinate ubiquinone oxidoreductase inhibitors. J Agric Food Chem 65:1021–1029. https://doi.org/10.1021/acs.jafc.6b05134
Acknowledgements
This work was financially supported by Young Top-Notch Talent Support Program of Guizhou Provincial Education Department (No. 2018038) and National Natural Science Foundation of China (No. 21362003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, Z., Shi, J., Luo, N. et al. Synthesis, crystal structure and antimicrobial activity of 2-((2-(4-(1H-1,2,4-triazol-1-yl)phenyl)quinazolin-4-yl)oxy)-N-phenylacetamide derivatives against phytopathogens. Mol Divers 23, 615–624 (2019). https://doi.org/10.1007/s11030-018-9896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9896-2