Abstract
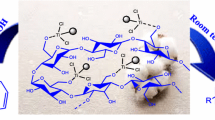
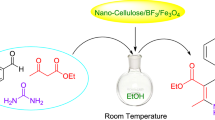
The present study describes an efficient and environmentally benign protocol for the synthesis of 2,3-dihydro-2-substituted-1H-naphtho[1,2-e][1,3]oxazine derivatives. Synthesis is done by one-pot three-component condensation of 2-naphthol, formaldehyde, and primary amines in the presence of Fe3O4@nano-cellulose/TiCl as a bio-based magnetic nano-catalyst under solvent-free conditions at room temperature grinding. This green procedure offers the advantages of high yield, short reaction time, environmental friendliness, easy workup, simple magnetic recovery of the catalyst and its reusability up to ten times without significant loss of catalytic activity. The structure of products was approved by FT-IR, 1H-NMR, 13C-NMR spectroscopy.
Graphical abstract




Similar content being viewed by others
References
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, New York
Kamitori Y, Hojo M, Masuda R, Kimura T, Yoshida T (1986) Selective protection of carbonyl compounds. Silica gel treated with thionyl chloride as an effective catalyst for thioacetalization. J Org Chem 51:1427–1431
Patney HK (1994) Anhydrous cobalt (II) bromide dispersed on silica gel: a mild and efficient reagent for thioacetalisation of carbonyl compounds. Tetrahedron Lett 35:5717–5718
Khan AT, Parvin T, Choudhury LH (2006) Silica-supported perchloric acid (HClO4-SiO2): a versatile catalyst for tetrahydropyranylation, oxathioacetalization and thioacetalization. Synthesis 2006:2497–2502
Mirjalili BBF, Bamoniri A, Akbari A (2008) BF3·SiO2: an efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo [a, j] xanthenes. Tetrahedron Lett 49:6454–6456
Davis RJ (2003) New perspectives on basic zeolites as catalysts and catalyst supports. J Catal 216:396–405
Mishra DK, Dabbawala AA, Hwang J-S (2013) Ruthenium nanoparticles supported on zeolite Y as an efficient catalyst for selective hydrogenation of xylose to xylitol. J Mol Catal A Chem 376:63–70
Rodriguez-Reinoso F (1998) The role of carbon materials in heterogeneous catalysis. Carbon 36:159–175
Wang X, Liu R, Waje MM, Chen Z, Yan Y, Bozhilov KN, Feng P (2007) Sulfonated ordered mesoporous carbon as a stable and highly active protonic acid catalyst. Chem Mater 19:2395–2397
Trueba M, Trasatti SP (2005) γ-Alumina as a support for catalysts: a review of fundamental aspects. Eur J Inorg Chem 2005:3393–3403
Solinas A, Taddei M (2007) Solid-supported reagents and catch-and-release techniques in organic synthesis. Synthesis 2007:2409–2453
Borujeni KP, Tamami B (2007) Polystyrene and silica gel supported AlCl3 as highly chemoselective heterogeneous Lewis acid catalysts for Friedel–Crafts sulfonylation of aromatic compounds. Catal Commun 8:1191–1196
Shaabani A, Maleki A (2007) Cellulose sulfuric acid as a bio-supported and recyclable solid acid catalyst for the one-pot three-component synthesis of α-amino nitriles. Appl Catal A Gen 331:149–151
Azad S, Mirjalili BBF (2017) Nano-TiCl 2/cellulose: an eco-friendly bio-based catalyst for one-pot synthesis of 4H-pyrimido [2, 1-b] benzothiazole derivatives. Res Chem Intermed 43:1723–1734
Lee M, Chen B-Y, Den W (2015) Chitosan as a natural polymer for heterogeneous catalysts support: a short review on its applications. Appl Sci 5:1272–1283
Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H (2009) Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J Mol Catal B Enzym. 58:103–109
Datta A, Adhikary J, Chatterjee S, Ghosh D, Khamarui S, Chattopadhyay T (2016) Synthesis and characterization of a magnetically separable novel Fe3O4@ L-DOPA@ CuII nanocatalyst (L-DOPA = L-3, 4-dihydroxyphenyylalanine): asymmetric aza-Michael addition reaction. Inorg Chim Acta 444:209–216
Zirak M, Jamali Garegeshlagi E (2018) Picolinimidoamide-Cu (II) complex anchored on Fe3O4@ SiO2 core–shell magnetic nanoparticles: an efficient reusable catalyst for click reaction. J Coord Chem 71:1–12
Yang H-H, Zhang S-Q, Chen X-L, Zhuang Z-X, Xu J-G, Wang X-R (2004) Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal Chem 76:1316–1321
Lu Y, Lu X, Mayers BT, Herricks T, Xia Y (2008) Synthesis and characterization of magnetic Co nanoparticles: a comparison study of three different capping surfactants. J Solid State Chem 181:1530–1538
Liu B, Zhang W, Yang F, Feng H, Yang X (2011) Facile method for synthesis of Fe3O4@ polymer microspheres and their application as magnetic support for loading metal nanoparticles. J Phys Chem C 115:15875–15884
Rostamnia S, Gholipour B, Liu X, Wang Y, Arandiyan H (2018) NH2-coordinately immobilized tris(8-quinolinolato)iron onto the silica coated magnetite nanoparticle: Fe3O4@SiO2-FeQ3 as a selective Fenton-like catalyst for clean oxidation of sulfides. J Colloid Interf Sci 511:447–455
Zhao J, Li C, Liu R (2018) Enhanced oxygen reduction of multi-Fe3O4@carbon core-shell electrocatalysts through a nanoparticle/polymer co-assembly strategy. Nanoscale 10:5882–5887
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Kuo C-H, Liu Y-C, Chang C-MJ, Chen J-H, Chang C, Shieh C-J (2012) Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohyd Polym 87:2538–2545
Li G, Jiang Y, Huang K, Ding P, Chen J (2008) Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J Alloys Compd 466:451–456
Malcolm R Jr, Saxena IM (2007) Cellulose: molecular and structural biology: selected articles on the synthesis, structure, and applications of cellulose. Springer, Berlin
Kadla JF, Gilbert RD (2000) Cellulose structure: a review. Cellul Chem Technol 34:197–216
Chavan PV, Pandit KS, Desai UV, Kulkarni MA, Wadgaonkar PP (2014) Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): a new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted–1,2,3-triazoles in water. RSC Adv 4:42137–42146
Azad S, Mirjalili BBF (2016) Fe3O4@ nano-cellulose/TiCl: a bio-based and magnetically recoverable nano-catalyst for the synthesis of pyrimido [2,1-b] benzothiazole derivatives. RSC Adv 6:96928–96934
Chylińska J, Urbański T, Mordarski M (1963) Dihydro-1,3-oxazine derivatives and their antitumor activity. J Med Chem 6:484–487
Mathew BP, Kumar A, Sharma S, Shukla PK, Nath M (2010) An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo- and naphtho-1,3-oxazine derivatives. Eur J Med Chem 45:1502–1507
Cocuzza AJ, Chidester DR, Cordova BC, Jeffrey S, Parsons RL, Bacheler LT, Erickson-Viitanen S, Trainor GL, Ko SS (2001) Synthesis and evaluation of efavirenz (SustivaTM) analogues as HIV-1 reverse transcriptase inhibitors: replacement of the cyclopropylacetylene side chain. Bioorg Med Chem Lett 11:1177–1179
Kurz T (2005) Synthesis of novel pyrido[2,3-e][1,3]oxazines. Tetrahedron 61:3091–3096
Kajino M, Shibouta Y, Nishikawa K, Meguro K (1991) Synthesis and biological activities of new 2-substituted 1,4-benzoxazine derivatives. Chem Pharm Bull 39:2896–2905
Buckman BO, Mohan R, Koovakkat S, Liang A, Trinh L, Morrissey MM (1998) Design, synthesis, and biological activity of novel purine and bicyclic pyrimidine factor Xa inhibitors. Bioorg Med Chem Lett 8:2235–2240
Katsura Y, Nishino S, Takasugi H (1991) Studies on antiulcer drugs. I. Synthesis and antiuler activities of imidazo [1,2-a] pyridinyl-2-oxobenzoxazolidines-3-oxo-2H-1,4-benzoxazines and related compounds. Chem Pharm Bull 39:2937–2943
Joyce JN, Presgraves S, Renish L, Borwege S, Osredkar T, Hagner D, Replogle M, PazSoldan M, Millan MJ (2003) Neuroprotective effects of the novel D3/D2 receptor agonist and antiparkinson agent, S32504, in vitro against 1-methyl-4-phenylpyridinium (MPP+) and in vivo against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a comparison to ropinirole. Exp Neurol 184:393–407
Burke W, Murdock KJ (1954) Condensation of hydroxyaromatic compounds with formaldehyde and primary aromatic amines. Am Chem Soc 76:1677–1679
Tang Z, Zhu Z, Xia Z, Liu H, Chen J, Xiao W, Ou X (2012) Synthesis and fungicidal activity of novel 2,3-disubstituted-1,3-benzoxazines. Molecules 17:8174–8185
Dong Y, Sun J, Wang X, Xu X, Cao L, Hu Y (2004) Highly regioselective N-alkylation of nonracemic Betti base: a novel one-pot synthesis of chiral N-methyl-N-alkyl Betti bases. Tetrahedron Asymmetry 15:1667–1672
Okimoto M, Ohashi K, Yamamori H, Nishikawa S, Hoshi M, Yoshida T (2012) Electrooxidative cyclization of hydroxyamino compounds possessing a benzyl group. Synthesis 44:1315–1322
Sadaphal SA, Sonar SS, Shingate BB, Shingare MS (2010) Water mediated synthesis of various [1,3] oxazine compounds using alum as a catalyst. Green Chem Lett Rev 3:213–216
Kategaonkar AH, Sonar SS, Pokalwar RU, Kategaonkar AH, Shingate BB, Shingare MS (2010) An efficient synthesis of 3, 4-dihydro-3-substituted-2H-naphtho [2,1-e][1,3] oxazine derivatives catalyzed by zirconyl (IV) chloride and evaluation of its biological activities. Bull Korean Chem Soc 31:1657–1660
Kategaonkar AH, Sonar SS, Shelke KF, Shingate BB, Shingare MS (2010) Ionic liquid catalyzed multicomponent synthesis of 3, 4-dihydro-3-substituted-2H-naphtho [2,1-e][1,3] oxazine derivatives. Org Commun 3:1–7
Shinde PV, Kategaonkar AH, Shingate BB, Shingare MS (2011) Polyethylene glycol (PEG) mediated expeditious synthetic route to 1,3-oxazine derivatives. Chin Chem Lett 22:915–918
Dhakane VD, Gholap SS, Deshmukh UP, Chavan HV, Bandgar BP (2014) An efficient and green method for the synthesis of [1,3] oxazine derivatives catalyzed by thiamine hydrochloride (VB1) in water. CR Chim 17:431–436
Shen AY, Tsai CT, Chen C-L (1999) Synthesis and cardiovascular evaluation of N-substituted 1-aminomethyl-2-naphthols. Eur J Med Chem 34:877–882
Woodgate PD, Horner GM, Maynard NP, Rickard CE (1999) Synthesis of dioxazaborocines from N-substituted-bis (2-hydroxyaryl) aminomethylamines. J Organomet Chem 592:180–193
Acknowledgements
The Research Council of Yazd University gratefully acknowledged for the financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azad, S., Mirjalili, B.B.F. One-pot solvent-free synthesis of 2,3-dihydro-2-substituted-1H-naphtho[1,2-e][1,3]oxazine derivatives using Fe3O4@nano-cellulose/TiCl as a bio-based and recyclable magnetic nano-catalyst. Mol Divers 23, 413–420 (2019). https://doi.org/10.1007/s11030-018-9884-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9884-6