Abstract
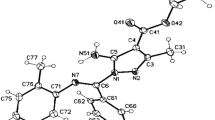
A general method for the synthesis of 1,3,5-trisubstituted 1,2,4-triazoles has been developed from reaction of amidrazones with ethyl azodicarboxylate and triethylamine (Mitsunobu reagent) in EtOH. This highly regioselective one-pot process provides rapid access to highly diverse triazoles. The reaction was explained, based on Mitsunobu reagent oxidizing ethanol to acetaldehyde, which would then react with amidrazones to give the substituted 3-methyltriazoles. A [2 + 3] cycloaddition reaction between two oxidized forms of amidrazones produced the second type of triazoles. X-ray structure analyses proved the structure of each type of product.
Graphical abstract






Similar content being viewed by others
References
Curtis ADM, Jennings N (2008) 1,2,4-triazoles. Compr Heterocycl Chem III 5:159–209. https://doi.org/10.1016/B978-008044992-0.00502-2
Zhou C-H, Wang Y (2012) Recent researches in triazole compounds as medicinal drugs. Curr Med Chem 19:239–280. https://doi.org/10.2174/092986712803414213
Shalini K, Kumar N, Drabu S, Sharma PK (2011) Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J Org Chem 7:668–677. https://doi.org/10.3762/bjoc.7.79
Turan-Zitouni G, Kaplancikli ZA, Yildiz MT, Chevallet P, Kaya D (2005) Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur J Med Chem 40:607–613. https://doi.org/10.1016/j.ejmech.2005.01.007
Walczak K, Gondela A, Suwinski J (2004) Synthesis and anti-tuberculosis activity of N-aryl-C- nitroazoles. Eur J Med Chem 39:849–853. https://doi.org/10.1016/j.ejmech.2004.06.014
Holla BS, Poojary KN, Rao BS, Shivananda MK (2002) New bis-aminomercaptotriazoles and bis- triazolothiadiazoles as possible anticancer agents. Eur J Med Chem 37:511–517. https://doi.org/10.1016/S0223-5234(02)01358-2
Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A, Shafiee A (2004) Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4- oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett 14:6057–6059. https://doi.org/10.1016/j.bmcl.2004.09.072
Mhasalkar MY, Shah MH, Nikam ST, Ananthanarayanan KG, Deliwala CV (1970) 4-Alkyl-5-aryl-4H-1,2,4-triazole-3-thiols as hypoglycemic agents. J Med Chem 13:672–674. https://doi.org/10.1021/jm00298a021
Amir M, Shikha K (2004) Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino)phenyl]acetic acid derivatives. Eur J Med Chem 39:535–545. https://doi.org/10.1016/j.ejmech.2004.02.008
Mitsunobu O, Yamada Y (1967) Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull Chem Soc Jpn 40:2380–2382. https://doi.org/10.1246/bcsj.40.2380
Mitsunobu O (1981) The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1981:1–28. https://doi.org/10.1055/s-1981-29317
Aly AA, Ramadan M, Abd El-Aziz M, Bräse S, Brown AB, Fathy HM, Nieger M (2017) Regioselective synthesis of 5-aminopyrazoles from reactions of amidrazones with activated nitriles: NMR investigation and x-ray structural analysis. Chem Pap 71:1409–1417. https://doi.org/10.1007/s11696-017-0131-x
Aly AA, Ramadan M, Abd El-Aziz M, Bräse S, Brown AB, Fathy HM (2017) Selectivity of amidrazones towards activated nitriles—synthesis of new pyrazoles and NMR investigation. Arkivoc. https://doi.org/10.24820/ark.5550190.p009.877
Aly AA, Hassan AA, Bräse S, Gomaa MA-M, Nemr FM (2017) Reaction of amidrazones with diaminomaleonitrile: synthesis of 4-amino-5-iminopyrazoles. J Heterocycl Chem 54:480–483. https://doi.org/10.1002/jhet.2607
Aly AA, Hassan AA, Gomaa MA-M, Bräse S, Nemr FM (2017) Reaction of amidrazones with phthaloyl chloride—synthesis of 1,2,4-triazolium salts (part I). J Heterocycl Chem 54:775–779. https://doi.org/10.1002/jhet.2643
Aly AA, Gomaa MAM, NourEl- Din AM, Fahmi MS (2006) Amidrazones in the synthesis of 1H-1,2,4-triazoles. Z Naturforsch 61b:1239–1242. https://doi.org/10.1515/znb-2006-1009
Aly AA, Brown AB, Shawky AM (2017) One-pot reaction of amidrazones, phthaloyl chloride, and triethyl amine: synthesis of (1′,2′,4′-triazole)-2-benzoic acid. J Heterocycl Chem 54:2375–2379. https://doi.org/10.1002/jhet.2829
Aly AA, Shaker RM (2005) 5-Benzyl-1H-tetrazoles from the reaction of 1-aryl-5-methyl-1H-tetrazoles with 1,2-dehydrobenzene. Tetrahedron Lett 46:2679–2682. https://doi.org/10.1016/j.tetlet.2005.02.072
Aly AA, Ramadan M, Abd Al-Aziz M, Fathy HM, Bräse S, Brown AB, Nieger M (2016) Reaction of amidrazones with 2,3-diphenylcyclopropenone: synthesis of 3-(aryl)-2,5,6-triphenylpyrimidin-4(3H)-ones. J Chem Res 40:637–639. https://doi.org/10.3184/174751916x14743924
Aly AA, Ramadan M, Morsy NM, Elkanzi NAA (2017) Inclusion of carbonyl groups of benzo[b]thiophene-2,5-dione into amidrazones: synthesis of 1,2,4-triazine-5,6-diones. J Heterocycl Chem 54:2067–2070. https://doi.org/10.1002/jhet.2805
Aly AA, Gomaa MAM, NourEl-Din AM, Fahmy MS (2007) Reactions of amidrazones with 1,4-quinones. Arkivoc 16:41–50. https://doi.org/10.3998/ark.5550190.0008.g04
Aly AA, NourEl-Din AM, Gomaa MA-M, Fahmy MS (2008) Rapid and facile synthesis of 4-aryl-5-imino-3-phenyl-1H-naphtho[2,3-f]-1,2,4-triazepine-6,11-diones via the reaction of amidrazones with dicyanonaphthoquinone. Z Naturforschung 63B:223–228. https://doi.org/10.1515/znb-2008-0217
Aly AA, Ishak EA, Ramadan M, Elkanzi NAA, El-Reedy AAM (2017) Amidrazones and 2-acetylcyclopentanone in the synthesis of cyclopenta[e][1,3,4]oxadiazepines. J Heterocycl Chem 54:1652–1655. https://doi.org/10.1002/jhet.2727
Nájera C, Sansano JM, Yus M (2015) 1,3-Dipolar cycloadditions of azomethine imines. Org Biomol Chem 13:8596–8636. https://doi.org/10.1039/C5OB01086A
Yoneda F, Suzuki K, Nitta Y (1966) A new hydrogen-abstracting reaction with diethyl azodicarboxylate. J Am Chem Soc 88:2328–2829. https://doi.org/10.1021/ja00962a05
Hayashi M, Shibuya M, Iwabuchi Y (2011) Oxidation of alcohols to carbonyl compounds with diisopropyl azodicarboxylate catalyzed by nitroxyl radicals. J Org Chem 77:3005–3009. https://doi.org/10.1021/jo300088b
Cao HT, Grée R (2009) DEAD-(cat)ZnBr2, an efficient system for the oxidation of alcohols to carbonyl compounds. Tetrahedron Lett 50:1493–1494. https://doi.org/10.1016/j.tetlet.2009.01.080
Shi M, Zhao G-L (2004) Aza-Baylis–Hillman reactions of diisopropyl azodicarboxylate or diethyl azodicarboxylate with acrylates and acrylonitrile. Tetrahedron 60:2083–2089. https://doi.org/10.1016/j.tet.2003.12.059
Tsunoda T, Otsuka J, Yamamiya Y, Ito S (1994) N,N,N′,N′-Tetramethylazodicarboxamide (TMAD), a new versatile reagent for Mitsunobu reaction. Its application to synthesis of secondary amines. Chem Lett 23:539–542. https://doi.org/10.1246/cl.1994.539
Tsunoda T, Kawamura Y, Uemoto K, Ito S (1998) Mitsunobu type C-N bond formation with 4,7-dimethyl-3,5,7-hexahydro-1,2,4,7-tetrazocin-3,8-dione (DHTD), a new cyclic azodicarboxamide. Heterocycles 47:177–179. https://doi.org/10.3987/com-97-s(n)78
Sakamoto I, Kaku H, Tsunoda T (2003) Preparation of (cyanomethylene)trimethylphosphorane as a new Mitsunobu-type reagent. Chem Pharm Bull 51:474–476. https://doi.org/10.1248/cpb.51.474
Hughes DL (2004) The Mitsunobu reaction. Org React (NY) 42:335–656. https://doi.org/10.1002/0471264180.or042.02
Jenkins ID, Mitsunobu O (2001) Triphenylphosphine-diethyl azodicarboxylate. In: Paquette LA (ed) Electronic encyclopedia of reagents for organic synthesis. Wiley, New York. https://doi.org/10.1002/047084289x.rt372
Müller D, Beckert R, Görls H (2001) Bis-amidines as useful building blocks for heterofulvenes and -fulvalenes. Synthesis 2001:601–606. https://doi.org/10.1055/s-2001-12366
Siddaiah V, Basha GM, Srinuvasarao R, Yadav SK (2011) HClO4-SiO2: an efficient reusable catalyst for the synthesis of 3,4,5-trisubstituted 1,2,4-triazoles under solvent-free conditions. Catal Lett 141:1511–1520. https://doi.org/10.1007/s10562-011-0665-4
Mangarao N, Mahaboob Basha G, Ramu T, Srinuvasarao R, Prasanthi S, Siddaiah V (2014) Brønsted acid-catalyzed simple and efficient synthesis of 1,2,4-triazoles and 1,2,4-oxadiazoles using 2,2,2-trichloroethyl imidates in PEG. Tetrahedron Lett 55:177–179. https://doi.org/10.1016/j.tetlet.2013.10.147
Ito S, Tanaka Y, Kakehi A, Miyazawa H (1977) The reaction of N-(phenylsulfonyl)-benzohydrazonoyl chloride with N-substituted benzamidines, with benzimidates, and with 2-aminopyridines. Bull Chem Soc Jpn 50:2969–2972. https://doi.org/10.1246/bcsj.50.2969
Nakka M, Tadikonda R, Nakka S, Vidavalur S (2016) Synthesis of 1,2,4-triazoles, N-fused 1,2,4-triazoles and 1,2,4-oxadiazoles via molybdenum hexacarbonyl-mediated carbonylation of aryl iodides. Adv Synth Catal 358:520–525. https://doi.org/10.1002/adsc.201500703
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3. https://doi.org/10.1107/S2053229614024218
Acknowledgements
The authors thank 3-MET Society, Karlsruhe Institute of Technology, Karlsruhe, Germany, for financial support to Prof Ashraf A. Aly enabling him to carry out analyses in the aforesaid Institute. Purchase of the NMR spectrometer at Florida Institute of Technology was assisted by the US National Science Foundation (CHE 03-42251).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aly, A.A., Hassan, A.A., Mohamed, N.K. et al. Regioselective formation of 1,2,4-triazoles by the reaction of amidrazones in the presence of diethyl azodicarboxylate and catalyzed by triethylamine. Mol Divers 23, 195–203 (2019). https://doi.org/10.1007/s11030-018-9868-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9868-6