Abstract
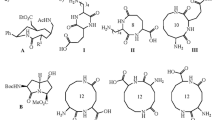
In this study, amino acids and peptides were used as reactants in a Hantzsch multicomponent reaction in order to synthesize new structurally diverse molecules containing these synthons. As well, an applicable strategy for modification of these natural molecules with heterocycle backbones such as pyrimidine, xanthene and acridine is introduced. Using this method, a set of new amino acid- and peptide-functionalized heterocycles were synthesized in good to excellent yields under mild conditions. Furthermore, carbohydrates were used as substrates in the synthesis of some derivatives. Overall, this methodology allows the possibility of synthesis of large numbers of natural product-based libraries, using amino acids, peptides and carbohydrates through combinatorial chemistry.
Graphical abstract






Similar content being viewed by others
References
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Small heterocycles in multicomponent reactions. Chem Rev 114:8323–8356. https://doi.org/10.1021/cr400615v
González-López M, Shaw JT (2009) Cyclic anhydrides in formal cycloadditions and multicomponent reactions. Chem Rev 109:164–189. https://doi.org/10.1021/cr8002714
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. https://doi.org/10.1021/cr0505728
Brauch S, van Berkel SS, Westermann B (2013) Higher-order multicomponent reactions: beyond four reactants. Chem Soc Rev 42:4948–4962. https://doi.org/10.1039/c3cs35505e
Gorea RP, Rajput AP (2013) A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent Today 5:148–152. https://doi.org/10.1016/j.dit.2013.05.010
Dömling A, Wang W, Wang K (2012) Chemistry and biology of multicomponent reactions. Chem Rev 112:3083–3185. https://doi.org/10.1021/cr100233r
Estévez V, Villacampa M, Menéndez JC (2010) Multicomponent reactions for the synthesis of pyrroles. Chem Soc Rev 39:4402–4421. https://doi.org/10.1039/B917644F
de Graaff C, Ruijter E, Orru RVA (2012) Recent developments in asymmetric multicomponent reactions. Chem Soc Rev 41:3969–4009. https://doi.org/10.1039/c2cs15361k
Tejedor D, García-Tellado F (2007) Chemo-differentiating ABB′ multicomponent reactions. Privileged building blocks. Chem Soc Rev 36:484–491. https://doi.org/10.1039/B608164A
Touré BB, Hall DG (2005) Multicomponent reactions in the total synthesis of natural products. In: Zhu J, Bienaymé H (eds) multicomponent reactions. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Touré BB, Hall DG (2009) Natural product synthesis using multicomponent reaction strategies. Chem Rev 109:4439–4486. https://doi.org/10.1021/cr800296p
Koszytkowska-Stawińska M, Buchowicz W (2014) Multicomponent reactions in nucleoside chemistry. Beilstein J Org Chem 10:1706–1731. https://doi.org/10.3762/bjoc.10.179
Nourisefat M, Panahi F, Khalafi-Nezhad A (2014) Carbohydrates as a reagent in multicomponent reactions: one-pot access to a new library of hydrophilic substituted pyrimidine-fused heterocycles. Org Biomol Chem 12:9419–9426. https://doi.org/10.1039/C4OB01791A
Musawwer Khan M, Yousuf R, Khana S, Shafiullah S (2015) Recent advances in multicomponent reactions involving carbohydrates. RSC Adv 5:57883–57905. https://doi.org/10.1039/C5RA08059B
Voigt B, Linke M, Mahrwald R (2015) Multicomponent cascade reactions of unprotected carbohydrates and amino acids. Org Lett 17:2606–2609. https://doi.org/10.1021/acs.orglett.5b00887
Ricardo MG, Morales FE, Garay H, Reyes O, Vasilev D, WessjohannL A, Rivera DG (2015) Bidirectional macrocyclization of peptides by double multicomponent reactions. Org Biomol Chem 13:438–446. https://doi.org/10.1039/C4OB01915F
Khalafi-Nezhad A, Divar M, Panahi F (2013) Nucleosides as reagents in multicomponent reactions: one-pot synthesis of heterocyclic nucleoside analogues incorporating pyrimidine-fused rings. Tetrahedron Lett 54:220–222. https://doi.org/10.1016/j.tetlet.2012.11.003
Toobaei Z, Yousefi R, Panahi F, Shahidpour S, Nourisefat M, Doroodmand MM, Khalafi-Nezhad A (2015) Synthesis of novel poly-hydroxyl functionalized acridine derivatives as inhibitors of α-Glucosidase and α-Amylase. Carbohydr Res 411:22–32. https://doi.org/10.1016/j.carres.2015.04.005
Krishna PR, Reddy PS (2008) “diversity oriented synthesis” of functionalized chiral tetrahydropyridines: potential GABA receptor agonists and azasugars from natural amino acids via a sequential Baylis–Hillman reaction and RCM protocol. J Comb Chem 10:426–435. https://doi.org/10.1021/cc700171p
Boyle AL, Woolfson DN (2011) De novo designed peptides for biological applications. Chem Soc Rev 40:4295–4306. https://doi.org/10.1039/C0CS00152J
Koopmanschap G, Ruijter E, OrruR VA (2014) Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J Org Chem 10:544–598. https://doi.org/10.3762/bjoc.10.50
Kimmerlin T, Seebach D (2005) 100 years of peptide synthesis: ligation methods for peptide and protein synthesis with applications to b-peptide assemblies. J Pept Res 65:229–260. https://doi.org/10.1111/j.1399-3011.2005.00214.x
Sokolova NV, Nenajdenko VG, Sokolov VB, Vinogradova DV, Shevtsova EF, DubovaL G, BachurinS O (2014) Synthesis and biological activity of N-substituted-tetrahydro-γ-carbolines containing peptide residues. Beilstein J Org Chem 10:155–162. https://doi.org/10.3762/bjoc.10.13
Grauer A, König B (2009) Peptidomimetics—a versatile route to biologically active compounds. Eur J Org Chem 2009:5099–5111. https://doi.org/10.1002/ejoc.200900599
Cini E, Bifulco G, Menchi G, Rodriquez M, Taddei M (2012) Synthesis of enantiopure 7-substituted azepane-2-carboxylic acids as templates for conformationally constrained peptidomimetics. Eur J Org Chem 2012:2133–2141. https://doi.org/10.1002/ejoc.201101387
Burgess K (2001) Solid-phase syntheses of β-turn analogues to mimic or disrupt protein–protein interactions. Acc Chem Res 34:826–835. https://doi.org/10.1021/ar9901523
Katritzky AR, Munawar MA, Kovacs J, Khelashvili L (2009) Synthesis of amino acid derivatives of quinolone antibiotics. Org Biomol Chem 7:2359–2362. https://doi.org/10.1039/B900762H
Portlock DE, Naskar D, West L, Ostaszewskic R, Chena JJ (2003) Solid-phase synthesis of five-dimensional libraries via a tandem Petasis–Ugi multi-component condensation reaction. Tetrahedron Lett 44:5121–5124. https://doi.org/10.1016/S0040-4039(03)01119-5
Wipf P, Wang X (2002) Parallel synthesis of oxazolines and thiazolines by tandem condensation-cyclodehydration of carboxylic acids with amino alcohols and aminothiols. J Comb Chem 4:656–660. https://doi.org/10.1021/cc020041m
Rottger S, Sjoberg PJR, Larhed M (2007) Microwave-enhanced copper-catalyzed N-arylation of free and protected amino acids in water. J Comb Chem 9:204–209. https://doi.org/10.1021/cc060150r
Liu Q, Yang H, Jiang Y, Zhao Y, Fu H (2013) General and efficient copper-catalyzed aerobic oxidative synthesis of N-fused heterocycles using amino acids as the nitrogen source. RSC Adv 3:15636–15644. https://doi.org/10.1039/c3ra41644e
Richter C, Trung MN, Mahrwald R (2015) Multicomponent cascade reactions of unprotected ketoses and amino acids—access to a defined configured quaternary stereogenic center. J Org Chem 80:10849–10865. https://doi.org/10.1021/acs.joc.5b02003
Yousefi A, Yousefi R, Panahi F, Sarikhani S, Zolghadr AR, Bahaoddini A, Khalafi-Nezhad A (2015) Novel curcumin-based pyrano[2,3-d]pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: implications for their pleiotropic effects against diabetes complications. Int J Biol Macromol 78:46–55. https://doi.org/10.1016/j.ijbiomac.2015.03.060
Suchý M, Hudson RHE (2014) Pyrimidine-fused heterocyclic frameworks based on an N4-arylcytosine scaffold: synthesis, characterization, and PNA oligomerization of the fluorescent cytosine analogue 5,6-BenzopC. J Org Chem 79:3336–3347. https://doi.org/10.1021/jo402873e
Yousefi R, Alavian-Mehr MM, Mokhtari F, Panahi F, Mehraban MH, Khalafi-Nezhad A (2013) Pyrimidine-fused heterocycle derivatives as a novel class of inhibitors for α-glucosidase.J. Enzyme Inhib Med Chem 28:1228–1235. https://doi.org/10.3109/14756366.2012.727812
Kumar PM, Kumar KS, Mohakhud PK, Mukkanti K, Kapavarapu R, Parsac KVL, Pal M (2012) Construction of a six-membered fused N-heterocyclic ring via a new 3-component reaction: synthesis of (pyrazolo) pyrimidines/pyridines. Chem Commun 48:431–433. https://doi.org/10.1039/C1CC16418J
Yan Z-F, Quan Z-J, Da Y-X, Zhang Z, Wang X-C (2014) A domino desulfurative coupling–acylation–hydration–Michael addition process for the synthesis of polysubstituted tetrahydro-4H-pyrido[1,2-a]pyrimidines. Chem Commun 50:13555–13558. https://doi.org/10.1039/C4CC05090H
Mohammadizadeh MR, Bahramzadeh M, Taghavi SZ (2010) A novel one-pot and efficient procedure for the synthesis of 3H-spiro[isobenzofuran-1,6′-pyrrolo[2,3-d]pyrimidine]-2′,3,4′,5′-tetraone. Tetrahedron Lett 51:5807–5809. https://doi.org/10.1016/j.tetlet.2010.08.113
Memarzadeh R, Noh H-B, Javadpour S, Panahi F, Feizpour A, Shim Y-B (2013) Carbon monoxide sensor based on a B2HDDT-doped PEDOT:PSS layer. Bull Korean Chem Soc 34:2291–2296. https://doi.org/10.5012/bkcs.2013.34.8.2291
Panahi F, Yousefi R, Mehraban MH, Khalafi-Nezhad A (2013) Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic a-glucosidase inhibitors. Carbohydr Res 380:81–91. https://doi.org/10.1016/j.carres.2013.07.008
Niknam K, Panahi F, Saberi D, Mohagheghnejad M (2010) Silica-bonded S-sulfonic acid as recyclable catalyst for the synthesis of 1,8-dioxo-decahydroacridines and 1,8-dioxo-octahydroxanthenes. J Heterocycl Chem 47:292–300. https://doi.org/10.1002/jhet.303
Verma C, Ebenso EE, Olasunkanmi LO, Quraishi MA, Obot IB (2016) Adsorption behavior of glucosamine based pyrimidine fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120:11598–11611. https://doi.org/10.1021/acs.jpcc.6b04429
Dömling A, Wang W, Wang K (2012) Chemistry and biology of multicomponent reactions. Chem Rev 112:3083–3135. https://doi.org/10.1021/cr100233r
Brahmachari G, Nurjamal K, Karmakar I, Begam S, Nayek N, Mandal B (2017) Development of a water-mediated and catalyst-free green protocol for easy access of a huge array of diverse and densely functionalized pyrido[2,3-d:6,5-d′]dipyrimidines via one-pot multicomponent reaction under ambient conditions. ACS Sustainable Chem Eng 5:9494–9505. https://doi.org/10.1021/acssuschemeng.7b02696
Ghahremanzadeh R, Fereshtehnejad F, Bazgir A (2010) Chromeno[2,3-d]pyrimidine-triones synthesis by a three-component coupling reaction. Chem Pharm Bull 58:516–520. https://doi.org/10.1248/cpb.58.516
To QH, LeeY R, Kim SH (2012) Efficient one-pot synthesis of acridinediones by indium(III) triflate-catalyzed reactions of β-enaminones, aldehydes, and cyclic 1,3-dicarbonyls. Bull Korean Chem Soc 33:1170–1176. https://doi.org/10.5012/bkcs.2012.33.4.1170
Sharma D, Bandna Reddy CB, Kumar S, Shil AK, Guha NR, Das P (2013) Cyclohexyl iodide promoted approach for coumarin analog synthesis using small scaffold. RSC Adv 17:651–659. https://doi.org/10.1007/s11030-013-9461-y
Crocker K (2012) Chemistry of carboxylic acid. Research World, Delhi
Tang Z-Q, Chen Y, Liu C-N, Cai K-Y, Tu S-J (2010) A green procedure for the synthesis of 1,8-dioxodecahydroacridine derivatives under microwave irradiation in aqueous media without catalyst. Heterocycl Chem 47:363–367. https://doi.org/10.1002/jhet.322
Tu S, Wang Q, Zhang Y, Xu J, Zhang J, Zhu X, Shi F (2006) An efficient one-pot synthesis of N-carboxymethylacridine-1,8-dione derivatives under microwave irradiation. J Heterocycl Chem 43:1647–1651. https://doi.org/10.1002/jhet.5570430633
Mokhtary M, Mirfarjood Langroudi SA (2014) Polyvinylpolypyrrolidone-supported boron trifluoride: a mild and efficient catalyst for the synthesis of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines. Monatsh Chem 145:1489–1494. https://doi.org/10.1007/s00706-014-1206-9
El-Sabbagh OI, Rady HM (2009) Synthesis of new acridines and hydrazones derived from cyclic b-diketone for cytotoxic and antiviral evaluation. Eur J Med Chem 44:3680–3686. https://doi.org/10.1016/j.ejmech.2009.04.001
Pal A, Shrivastava S, Dey J (2009) Salt, pH and thermoresponsive supramolecular hydrogel of N-(4-n-tetradecyloxybenzoyl)-l-carnosine. Chem Commun. https://doi.org/10.1039/B914665B
Grasso GI, Bellia F, Arena G, Vecchio G, Rizzarelli E (2011) Noncovalent interaction-driven stereoselectivity of copper(II) complexes with cyclodextrin derivatives of l- and d-carnosine. Inorg Chem 50:4917–4924. https://doi.org/10.1021/ic200132a
Polshettiwar V, Baruwati B, Varma RS (2009) Magnetic nanoparticle-supported glutathione: a conceptually sustainable organocatalyst. Chem Commun. https://doi.org/10.1039/B900784A
Kim M, Ock K, ChoK JooS-W, LeeS Y (2012) Live-cell monitoring of the glutathione-triggered release of the anticancer drug topotecan on gold nanoparticles in serum-containing media. Chem Commun 48:4205–4207. https://doi.org/10.1039/C2CC30679D
He L, Xu Q, Liu Y, Wei H, Tang Y, Lin W (2015) A coumarin based turn-on fluorescence probe for specific detection of glutathione over cysteine and homocysteine. ACS Appl Mater Interfaces 7:12809–12813. https://doi.org/10.1021/acsami.5b01934
Patruno A, Fornasari E, Stefano AD, Cerasa LS, Marinelli L, Baldassarre L, Sozio P, Turkez H, Franceschelli S, Ferrone A, Giacomo VD, Speranza L, Felaco M, Cacciatore I (2015) Synthesis of a novel cyclic prodrug of S-allyl-glutathione able to attenuate LPS-induced ROS production through the inhibition of MAPK pathways in U937 cells. Mol Pharm 12:66–74. https://doi.org/10.1021/mp500431r
Khalafi-Nezhad A, Panahi F (2011) Synthesis of new dihydropyrimido [4, 5-b] quinolinetrione derivatives using a four-component coupling reaction. Synthesis 6:984–992. https://doi.org/10.1055/s-0030-1258446
Acknowledgements
The financial support from the Research Councils of Shiraz University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nourisefat, M., Panahi, F. & Khalafi-Nezhad, A. Amino acids and peptides as reactants in multicomponent reactions: modification of peptides with heterocycle backbones through combinatorial chemistry. Mol Divers 23, 317–331 (2019). https://doi.org/10.1007/s11030-018-9861-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9861-0