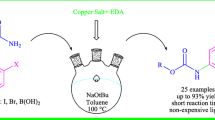
Abstract
A highly efficient, metal-free and solvent-free process is reported for the preparation of novel series of alkyl and aryl primary thiocarbamates and dithiocarbamates through the reaction of aliphatic alcohols, phenols and thiols with thiocyanate salts in the presence of 4-dodecylbenzenesulfonic acid (DBSA) as an inexpensive, readily available and amphipathic acid reagent. All reactions proceeded smoothly, and the products are obtained in good to excellent yields. Using this method, a wide range of structurally diverse primary thiocarbamates was prepared successfully.
Graphical Abstract




Similar content being viewed by others
References
Walter W, Bode KD (1967) Syntheses of thiocarbamates. Angew Chem Int Ed 6:281–293. https://doi.org/10.1002/anie.196702811
Spallarossa A, Cesarini S, Schenone S, Ranise A (2009) Parallel synthesis of O-phenoxyethyl and O-adamantyl N-acyl thiocarbamates endowed with antiproliferative activity. Arch Pharm 342:344–352. https://doi.org/10.1002/ardp.200800212
Rodis NP, Digenis GA (2001) Synthesis and in-vitro evaluation of novel low molecular weight thiocarbamates as inhibitors of human leukocyte elastase. J Enzyme Inhib 16:95–105. https://doi.org/10.1080/14756360109162359
Ramazonov NS, Syrov VN (2006) Synthesis of cyasteronylthiocarbamate derivatives. Chem Nat Compd 42:558–561. https://doi.org/10.1007/s10600-006-0213-4
Cesarini S, Spallarossa A, Ranise A, Fossa P, La Colla P, Sanna G, Collu G, Loddo R (2008) Thiocarbamates as non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 1: parallel synthesis, molecular modelling and structure-activity relationship studies on O-[2-(hetero) arylethyl]-N-phenylthiocarbamates. Bioorg Med Chem 16:4160–4172. https://doi.org/10.1016/j.bmc.2007.12.050
Crich D, Quintero L (1989) Radical chemistry associated with the thiocarbonyl group. Chem Rev 89:1413–1429. https://doi.org/10.1021/cr00097a001
Zard SZ (1997) On the trail of xanthates: some new chemistry from an old functional group. Angew Chem Int Ed 36:672–685. https://doi.org/10.1002/anie.199706721
Raghuvanshi RS (2015) A mild, efficient, and fast synthesis of methyl N-aryldithiocarbamates using superoxide ion at room temperature. Phosphorus Sulfur Silicon Relat Elem 190:133–137. https://doi.org/10.1080/10426507.2014.919292
Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Masini E, Supuran CT (2012) Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 55:1721–1730. https://doi.org/10.1021/jm300031j
Bahrin LG, Hopf H, Jones PG, Sarbu LG, Babii C, Mihai AC, Stefan M, Birsa LM (2016) Antibacterial structure-activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J Org Chem 12:1065–1071. https://doi.org/10.3762/bjoc.12.100
Rafin C, Veignie E, Sancholle M, Postel D, Len C, Villa P, Ronco G (2000) Synthesis and antifungal activity of novel bisdithiocarbamate derivatives of carbohydrates against fusarium oxysporum f. sp. lini. J Agric Food Chem 48:5283–5287. https://doi.org/10.1021/jf0003698
Grainger RS, Welsh EJ (2007) Formal synthesis of (-)-aphanorphine using sequential photomediated radical reactions of dithiocarbamates. Angew Chem 119:5473–5476. https://doi.org/10.1002/ange.200701055
Tsuboi S, Takeda S, Yamasaki Y, Sakai T, Utaka M, Ishida S, Yamada E, Hirano J (1992) A convenient synthesis of platelet-activating factors and their analogues from chiral epichlorohydrin. Chem Lett 21:1417–1420. https://doi.org/10.1246/cl.1992.1417
Katritzky AR, Singh S, Mohapatra PP, Clemens N, Kirichenko K, Molina P (2005) Synthesis of functionalized dithiocarbamates via N-(1-benzotriazolylalkyl) dithiocarbamates. Arkivoc 9:63–79
Chauhan R, Trivedi M, Yadav R, Kumar A, Amalnerkar DP, Gosavi SW (2015) Synthesis, characterization and light harvesting properties of Sb (III) and Bi (III) ferrocenyl dithiocarbamate complexes. Spectrochim Acta Mol Biomol Spectrosc 150:652–656. https://doi.org/10.1016/j.saa.2015.06.012
Trivedi M, Nagarajan R, Kumar A, Singh NK, Rath NP (2011) Synthesis, structure, catalytic and calculated non-linear optical properties of cis-and trans-, mer-chlorobis (triphenyl phosphine/triphenyl arsine)-dipicolinato ruthenium III complexes. J Mol Struct 994:29–38. https://doi.org/10.1016/j.molstruc.2011.02.044
Jing X, Liu F, Yang X, Ling P, Li L, Long C, Li A (2009) Adsorption performances and mechanisms of the newly synthesized N, N\(\prime \)-di (carboxymethyl) dithiocarbamate chelating resin toward divalent heavy metal ions from aqueous media. J Hazard Mater 167:589–596. https://doi.org/10.1016/j.jhazmat.2009.01.020
Bai L, Hu H, Fu W, Wan J, Cheng X, Zhuge L, Xiong L, Chen Q (2009) Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater 195:261–275. https://doi.org/10.1016/j.jhazmat.2011.08.038
Wuts PG, Greene TW (2006) Greene’s protective groups in organic synthesis. Wiley, Hoboken
Alam MN, Mandal SK, Debnath SC (2012) Effect of zinc dithiocarbamates and thiazole-based accelerators on the vulcanization of natural rubber. Rubber Chem Technol 85:120–131. https://doi.org/10.5254/1.3672434
Singh V, Kumar V, Gupta AN, Drew MG, Singh N (2014) Effect of pyridyl substituents leading to the formation of green luminescent mercury (II) coordination polymers, zinc (II) dimers and a monomer. New J Chem 38:3737–3748. https://doi.org/10.1039/C4NJ00435C
Weber WP, Gokel GW, Ugi IK (1972) Phasenübergangs-Katalyse bei der Hofmannschen Carbylamin–Reaktion. Angew Chem 84:587–587. https://doi.org/10.1002/ange.19720841211
Walter W, Bode KD (1966) Über die Oxydationsprodukte von Thiocarbonsäureamiden, XV. Oxydation von Thiocarbamidsäure-O-arylestern zu ortho-substituierten Aryloxy-iminomethansulfensäuren. Eur J Org Chem 698:122–130. https://doi.org/10.1002/jlac.19666980114
Grzyb JA, Shen M, Yoshina-Ishii C, Chi W, Brown RS, Batey RA (2005) Carbamoylimidazolium and thiocarbamoylimidazolium salts: novel reagents for the synthesis of ureas, thioureas, carbamates, thiocarbamates and amides. Tetrahedron 61:7153–7175. https://doi.org/10.1016/j.tet.2005.05.056
Villemin D, Hachemi M, Lalaoui M (1996) Potassium fluoride on alumina: synthesis of O-aryl N, N-dimethylthiocarbamates and their rearrangement into S-aryl N, N-dimethyl-thiocarbamates under microwave irradiation. Synth Commun 26:2461–2471. https://doi.org/10.1080/00397919608004558
Lee AW, Chan WH, Wong HC, Wong MS (1989) One pot phase transfer synthesis of O-alkyl, S-methyl dithiocarbonates (xanthates). Synth Commun 19:547–552. https://doi.org/10.1080/00397918908050698
Bararjanian M, Balalaie S, Rominger F, Movassagh B, Bijanzadeh HR (2011) Novel and efficient one-pot five-and six-component reactions for the stereoselective synthesis of highly functionalized enaminones and dithiocarbamates. Mol Divers 15:583–594. https://doi.org/10.1007/s11030-010-9286-x
Wood MR, Duncalf DJ, Rannard SP, Perrier S (2006) Selective one-pot synthesis of trithiocarbonates, xanthates, and dithiocarbamates for use in RAFT/MADIX living radical polymerizations. Org Lett 8:553–556. https://doi.org/10.1021/ol0525617
Bhadra S, Saha A, Ranu BC (2008) One-pot copper nanoparticle-catalyzed synthesis of S-aryl-and S-vinyl dithiocarbamates in water: high diastereoselectivity achieved for vinyl dithiocarbamates. Green Chem 10:1224–1230. https://doi.org/10.1039/B809200A
Azizi N, Aryanasab F, Tourkian L, Saidi MR (2010) Versatile and Large-Scale Synthesis of Functional Dithiocarbamates in Water. Synth Commun 41:94–99. https://doi.org/10.1080/00397910903531847
Halimehjani AZ, Marjani K, Ashouri A (2010) Synthesis of dithiocarbamate by Markovnikov addition reaction in aqueous medium. Green Chem 12:1306–1310. https://doi.org/10.1039/C004711B
Chaturvedi D, Ray S (2006) An efficient, one-pot, synthesis of dithiocarbamates from the corresponding alcohols using Mitsunobu’s reagent. Tetrahedron Lett 47:1307–1309. https://doi.org/10.1016/j.tetlet.2005.12.079
Liu Y, Bao W (2007) A new method for the synthesis of dithiocarbamates by CuI-catalyzed coupling reaction. Tetrahedron Lett 48:4785–4788. https://doi.org/10.1016/j.tetlet.2007.03.168
Ranu BC, Saha A, Banerjee S (2008) Catalysis by Ionic Liquids: Significant Rate Acceleration with the Use of [pmIm] Br in the Three-Component Synthesis of Dithiocarbamates. Eur J Org Chem 2008:519–523. https://doi.org/10.1002/ejoc.200700842
Sureshkumar D, Koutha SM, Chandrasekaran S (2005) Chemistry of tetrathiomolybdate: aziridine ring opening reactions and facile synthesis of interesting sulfur heterocycles. J Am Chem Soc 127:12760–12761. https://doi.org/10.1021/ja052969z
Stamm H (1999) Nucleophilic ring opening of aziridines. Adv Synth Catal 341:319–331. 10.1002/(SICI)1521-3897(199905)341:4\(<\)319::AID-PRAC319\(>\)3.0.CO;2-9
Hu XE (2004) Nucleophilic ring opening of aziridines. Tetrahedron 60:2701–2743. https://doi.org/10.1016/j.tet.2004.01.042
Modarresi-Alam AR, Inaloo ID, Kleinpeter E (2012) Synthesis of primary thiocarbamates by silica sulfuric acid as effective reagent under solid-state and solution conditions. J Mol Struct 1024:156–162. https://doi.org/10.1016/j.molstruc.2012.05.033
Sardarian AR, Inaloo ID (2015) 4-Dodecylbenzenesulfonic acid (DBSA) promoted solvent-free diversity-oriented synthesis of primary carbamates, S-thiocarbamates and ureas. RSC Adv 5:76626–76641. https://doi.org/10.1039/C5RA14528G
Crowell TI, Hankins MG (1969) Hydrolysis of thiocyanic acid. I. Dependence of rate on acidity function. J Phys Chem 73:1380–1383. https://doi.org/10.1021/j100725a035
Bowman WR, Burchell CJ, Kilian P, Slawin AM, Wormald P, Woollins JD (2006) Investigations on organo-sulfur-nitrogen rings and the thiocyanogen polymer(SCN) x. Chem Eur J 12:6366–6381. https://doi.org/10.1002/chem.200501528
Cataldo F (1992) Possible structure of parathiocyanogen: an IR and UV study. Polyhedron 11:79–83. https://doi.org/10.1016/S0277-5387(00)83263-5
Cataldo F, Fiordiponti P (1993) Possible structure of parathiocyanogen–II. electrochemical synthesis, \(^{13}\)C NMR and conductivity measurements on undoped and iodine doped samples. Polyhedron 12:279–284. https://doi.org/10.1016/S0277-5387(00)81724-6
Acknowledgements
Authors gratefully acknowledge the financial support of this work by the Research Council of Shiraz University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sardarian, A.R., Inaloo, I.D. & Modarresi-Alam, A.R. Highly efficient synthesis of alkyl and aryl primary thiocarbamates and dithiocarbamates under metal- and solvent-free conditions. Mol Divers 22, 863–878 (2018). https://doi.org/10.1007/s11030-018-9831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9831-6