Abstract
Naphthoquinone amino derivatives exhibit interesting physicochemical properties and a wide range of biological activities with potential medicinal applications. A clean, fast and simple method for the preparation of phenylamino-1,4-naphthoquinones is presented by the reaction of naphthoquinone (NQ) and anilines under ultrasound irradiation (US). Anilino derivatives were synthesized in good yields and shorter reaction times in comparison with the conventional method. This ultrasound procedure can be applied to the preparation of naphthoquinone derivatives with anilines containing electron-donor substituents (2-OMe, 4-OMe, 4-Me and 4-OEt) or halogen or electron-withdrawing substituents (4-F, 4-Cl, 4-Br, 3-F, 3-Cl, 3-Br, 4-Ac). This procedure was also applied to the reaction of anilines with 2,3-dichloro-1,4-naphthoquinone (DCNQ). A reaction mechanism involving an EDA complex is proposed based on NMR experiments and previous studies about solid/solid reactions.
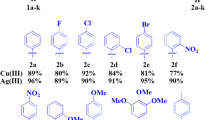
Graphical Abstract







Similar content being viewed by others
References
Riffel A, Medina LF, Stefani V, Santos RC, Bizani D, Brandelli A (2002) In vitro antimicrobial activity of a new series of 1,4-naphthoquinones. Braz J Med Biol Res 35:811–818. https://doi.org/10.1590/S0100-879X2002000700008
Ball MD, Bartlett MS, Shaw M, Smith JW, Nasr M, Meshnik SR (2002) Activities and conformational fitting of 1,4-naphthoquinone derivatives and other cyclic 1,4-diones tested in vitro against Pneumocystis carin. Agents Chemother 45:1473–1479. https://doi.org/10.1128/AAC.45.5.1473-1479.2001
Bittner S, Gorohovsky S, Lozinsky E, Shames AI (2000) EPR study of anion radicals of various N-quinonyl amino acids. Amino Acids 19:439–449. https://doi.org/10.1007/s007260070022
Medina LFC, Stefani V, Brandelli A (2006) An unusual reduction on the quinonoid ring of 5-amino-8-hydroxy-1,4-naphthoquinone by Staphylococcus aureus. Lett Appl Microbiol 42:381–385. https://doi.org/10.1111/j.1472-765x.2006.01863.x
Biot C, Bauer H, Schirmer RH, Davioud-Charret E (2004) 5-Substituted tetrazoles as bioisosteres of carboxylic acids. bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem 47:5972–5983. https://doi.org/10.1021/jm0497545
Mantyla A, Garnier-Rautio JT, Nevalainen T, Vepsalainen J, Koskinen A, Croft SI, Jarvinen T (2004) Synthesis, in vitro evaluation, and antileishmanial activity of water-soluble prodrugs of buparvaquone. J Med Chem 47:188–195. https://doi.org/10.1021/jm030868a
Tandon VK, Yadav DB, Maurya HK, Chaturvedi AK, Shukla PK (2006) Design, synthesis, and biological evaluation of 1,2,3-trisubstituted-1,4-dihydrobenzo[g]quinoxaline-5,10-diones and related compounds as antifungal and antibacterial agents. Bioorg Med Chem 14:6120–6126. https://doi.org/10.1016/j.bmc.2006.04.029
Leyva E, López LI, García de la Cruz RF, Espinosa-González CG (2016) Synthesis and studies of the antifungal activity of 2-anilino-/2,3-dianilino-/2-phenoxy- and 2,3-diphenoxy-1,4-naphthoquinones. Res Chem Int 43:1813–1827. https://doi.org/10.1007/s11164-016-2732-3
Kutyrev AA (1991) Nucleophilic reactions of quinones. Tetrahedron 47:8043–8065. https://doi.org/10.1016/s0040-4020(01)91002-6
Leyva E, Schmidke-Sobeck SJ, Loredo-Carrillo SE, Magaldi-Lara DA (2014) Spectral and structural characterization of 2-(fluorophenylamino)- and 2-(nitrophenylamino)-1,4-naphthoquinone derivatives. J Mol Struct 1068:1–7. https://doi.org/10.1016/j.molstruc.2014.03.044
Leyva E, López LI, Loredo-Carrillo SE, Rodríguez-Kessler M, Montes-Rojas A (2011) Synthesis, spectral and electrochemical characterization of novel 2-(fluoroanilino)-1,4-naphthoquinones. J Fluorine Chem 132:94–101. https://doi.org/10.1016/j.jfluchem.2010.12.001
Leyva E, López LI, Moctezuma E, de Lasa H (2008) A bentonitic clay assisted method for the preparation of 2-(R-anilino)-1, 4-naphthoquinones. Top Catal 49:281–287. https://doi.org/10.1007/s11244-008-9088-x
Leyva E, Baines KM, Espinosa-González CG, Magaldi-Lara DA, Loredo-Carrillo SE, de Luna-Méndez TA, López L (2015) 2-(Fluoro) and 2-(methoxyaniline)-1,4-naphthoquinones. Synthesis and mechanism and effect of fluorine substitution on redox reactivity and NRM. J Fluorine Chem 180:152–160. https://doi.org/10.1016/j.jfluchem.2015.08.016
Lisboa CS, Santos VG, Vaz BG, de Lucas NC, Eberlin MN, Garden SJ (2011) C–H functionalization of 1,4-naphthoquinone by oxidative coupling with anilines in the presence of a catalytic quantity of copper(II) acetate. J Org Chem 76:5264–5273. https://doi.org/10.1021/jo200354u
Aguilar-Martínez M, Cuevas G, Jiménez-Estrada M, González I, Lotina- Hennsen B, Macías-Ruvalcaba N (1999) An experimental and theoretical study of the substituent effects on the redox properties of 2-(R-phenyl)amino-1,4-naphthalenediones in acetonitrile. J Org Chem 64:3684–3694. https://doi.org/10.1021/jo990186o
Pokhrel N, Vabbina PK, Pala N (2016) Sonochemistry: science and engineering. Ultrason Sonochem 29:104–128. https://doi.org/10.1016/j.ultsonch.2015.07.023
Bretanha LC, Teixeira VE, Ritter M, Siqueira GM, Cunico W, Pereira CMP, Freitag RA (2011) Ultrasound-promoted synthesis of 3-trichloromethyl-5-alkyl(aryl)-1,2,4-oxadiazoles. Ultrason Sonochem 18:704–707. https://doi.org/10.1016/j.ultsonch.2010.09.016
Srivastava RM, Filho RAWN, da Silva CA, Bortoluzzi A (2009) First ultrasound-mediated one-pot synthesis of N-substituted amides. Ultrason Sonochem 16:737–742. https://doi.org/10.1016/j.ultsonch.2009.04.006
Duarte A, Cunico W, Pereira CMP, Freitag RA, Siqueira GM (2010) Ultrasound promoted synthesis of thioesters from 2-mercaptobenzoxa(thia)zoles. Ultrason Sonochem 17:281–283. https://doi.org/10.1016/j.ultsonch.2009.08.004
Neelgund GM, Kulkairni AS, Bundi ML (2004) EDA complexes of 2,3-dichloro-1,4-naphthoquinone with some substituted anilines. Monatsh Chem 135:343–355. https://doi.org/10.1007/s00706-003-0128-8
Nagendrappa RR, Jayadevappa ES (1975) Donor–acceptor equilibria involving para-benzoquinone and 1,4-naphthoquinone as acceptors and N-methyl-anilines and N,N-dimethyl-anilines as donors. Indian J Chem 13:1173–1176
Sieveking I, Thomas P, Estévez JC, Quiñones N, Cuéllar MA, Villena J, Espinosa-Bustos C, Fierro A, Tapia RA, Maya D, López-Muñoz R, Cassels BK, Estévez RJ, Salas CO (2014) 2-Phenylaminonaphthoquinones and related compounds: synthesis, trypanocidal and cytotoxic activities. Bioorg Med Chem 22:4609–4620. https://doi.org/10.1016/j.bmc.2014.07.030
Satheshkumar A, Kuppanagounder PE (2012) Spectroscopic and theoretical studies on the nucleophilic substitution of 2,3-dichloronaphthoquinone with para-substituted anilines in solid state via initial charge transfer complexation. Spectrochim Acta A 98:378–383. https://doi.org/10.1016/j.saa.2012.08.056
Prachayasittikul V, Pingaew R, Worachartcheewan A, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2014) Synthesis, anticancer activity and QSAR study of 1,4-naphthoquinone derivatives. Eur J Med Chem 84:247–263. https://doi.org/10.1016/j.ejmech.2014.07.024
Satheshkumar A, Ganesh K, Elango P (2014) Charge transfer facilitated direct electrophilic substitution in phenylaminonaphthoquinones: experimental, theoretical and electrochemical studies. New J Chem 38:993–1003. https://doi.org/10.1039/c3nj01228j
Kim YS, Park SY, Lee HJ, Suh ME, Schollmeyer D, Lee CO (2003) Synthesis and cytotoxicity of 6,11-dihydro-pyrido- and 6,11-dihydro-benzo[2,3-b]phenazine-6,11-dione derivatives. Bioorg Med Chem 11:1709–1714. https://doi.org/10.1016/s0968-0896(03)00028-2
Acknowledgements
Financial support by CONACyT (Grant No. 155678) is gratefully acknowledged. All authors kindly acknowledge The National Laboratory for Characterization of Physicochemical Properties and Molecular Structure, CONACyT (Grant NO. 123732) for the instrumentation time provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leyva, E., Cárdenas-Chaparro, A., Loredo-Carrillo, S.E. et al. Ultrasound-assisted reaction of 1,4-naphthoquinone with anilines through an EDA complex. Mol Divers 22, 281–290 (2018). https://doi.org/10.1007/s11030-018-9820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9820-9